Drinking Water Treatment and Disinfection
advertisement
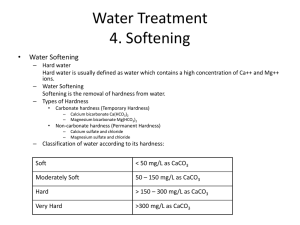
Disinfection – Chapter 26 Class Objectives • Be able to define the term disinfectant • List different types of disinfectants • List factors that influence disinfection • Be able to write the disinfection inactivation equation and how it relates to ideal and non-ideal disinfection behavior • Be able to define a C ▪ t value • List the common disinfectants used in drinking water and their advantages and disadvantages Disinfection • The destruction or prevention of growth of microorganisms capable of causing diseases • The final barrier against human exposure to pathogens • Disinfectants include: heat – denatures proteins and nucleic acids chemicals – uses a variety of mechanisms filtration – physical removal of a pathogen radiation – destroys nucleic acids • Some disinfectants also control taste and odor problems, organic matter, and metals such as iron and manganese Factors Influencing Disinfection • Type of disinfectant • Type of microorganism • Disinfectant concentration and time of contact • pH • Temperature • Chemical and physical interference, e.g., clumping of cells or adsorption to larger particles Cell-Mediated Mechanisms of Resistance to Disinfectants • Modification of sensitive/disinfectant action sites – enzymes • Cell wall/cell membrane alterations - allow reduced permeability • Cellular aggregation - provides physical protection • Capsule production - limits diffusion of disinfectant into cell Kinetics of Disinfection Inactivation is a gradual process involving a series of physicochemical and biochemical steps. Inactivation is described by the equation: Nt/N0 = e-kt 0 Shoulder Ideally, inactivation follows first-order kinetics (blue line), but often non-ideal behaviors occur resulting from clumping of cells or multiple hits of critical sites before inactivation Ideal, first order Rapid, initial inac tivation o Log (N t/N ) Where: N0 = number of microorganisms at time = 0 Nt = number of microorganisms at time = t k = a decay constant (1/time) t = time Ta iling o ff -x Time Concentration and Contact Time Effectiveness of chlorination depends primarily on the concentration used and the time of exposure Disinfectant effectiveness can be expressed as a C ▪ t value where: C = disinfectant concentration t = time required to inactivate a 99% of the population under specific conditions The lower the C ▪ t, the more effective the disinfectant In general, resistance to disinfection is in the following order: vegetative bacteria < enteric viruses < spore-forming bacteria < protozoan cysts Common Disinfectants in Water Treatment • Chlorine • Chloramines • Chlorine dioxide • Ozone • Ultraviolet light Chlorine • Most commonly used disinfectant • In water chlorine undergoes the following reaction: Cl2 + H2O HOCl HOCl + HCl H+ + OCl- • HOCl and OCl- is defined as free available chlorine • HOCl more effective than OCl- due to lack of charge • Presence of HOCL and OCl- is determined by pH • In drinking water 1 mg/L of chlorine for 30 min is generally sufficient to reduce bacterial numbers. In wastewater with interfering substances up to 20-40 mg/L may be required Interfering Substances • Turbidity can prevent adequate contact between chlorine and pathogens • Chlorine reacts with organic and inorganic nitrogenous compounds, iron, manganese, and hydrogen sulfide. • Dissolved organic compounds exert a chlorine demand • Knowing the concentrations of interfering substances is important in determining chlorine dose Chlorine inactivation of microorganisms results from: • Altered permeability of the outer cellular membrane, resulting in leakage of critical cell components • Interference with cell-associated membrane functions (e.g., phosphorylation of high-energy compounds • Impairment of enzyme and protein function as a result of irreversible binding of the sulfhydryl groups • Nucleic acid denaturation Chloramines Chloramines are produced by combining chlorine and ammonia NH3 + HOCl NH2 + H2O monochloramine NH2Cl + HOCl NH2Cl2 + H2O dichloramine NH2Cl2 + HOCl NCl3 + H2O trichloramine breakpoint reaction Used mainly as secondary disinfectants, e.g., following ozone treatment, when a residual in the distribution system is needed Chlorine Dioxide: ClO2 • Extreme soluble in water • Does not form trihalomethanes • Must be generated on-site: 2NaClO2 + Cl2 2ClO2 + 2NaCl Ozone: O3 • Very strong oxidant (very low C▪t values) but has no residual disinfection power • Generated by passing high voltage through the air between two electrodes • More expensive than chlorination but does not produce trihalomethanes which are suspected carcinogens • Widely used in Europe, limited use in U.S. Oxidant Advantages Disadvantages Chlorine Strong oxidant Persistant residual Chlorinated by-products Taste and odor problems pH influences effectiveness Chloramines No trihalomethane formation Persistant residual Weak oxidant Some organic halide formation Taste, odor, and growth problems Chlorine dioxide Strong oxidant Relatively persistant residual No trihalomethane prod. No pH effect Total organic halide formation ClO3 and ClO2 by products On-site generation required Hydrocarbon odors possible Ozone Strong oxidant No trihalomethane or organic halide formed No taste or odor prob. Little pH effects Coagulant aid Some by products biodegradable Short half-life On-site generation required Energy intensive Some by products biodegradable Complex generation Corrosive UV Disinfection • Optimum ultraviolet light wavelength range for germicidal effect: 250 nm - 270 nm • Low pressure mercury lamps emit 253.7 nm • Damages microbial/viral DNA and viral RNA by causing dimerization, blocking nucleic acid replication • Does not produce toxic by-products • Higher costs than chemical disinfection, no residual disinfection Repair of UV Damage in Bacteria • Photoreactivation -enzymatic repair (dimers are split) occurs under visible light (300-500nm) • Dark repair - excision of dimers