DISINFECTION: DRINKING WATER AND WASTEWATER
advertisement

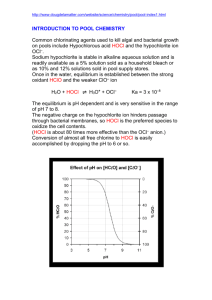
DISINFECTION: DRINKING WATER AND WASTEWATER Disinfection: destruction of pathogenic organisms Sterilization: destruction of all microorganisms Purpose: disinfect to prevent the transmission of water-borne diseases, i.e., destroy bacteria, viruses, amebic cysts Possible Disinfectants Physical: 1. Heat: boiling water; no important pathogens are heat resistant, costly 2. Ultraviolet light: no residual, thin sheets 3. Filtration: e.g., diatomaceous earth 4. Radiation: γ (gamma), ultrasonic, acoustic Possible Disinfectants, cont. Chemical: 5. Chemical agents a) chlorine b) bromine c) iodine oxidants d) ozone e) H2O2 f) phenol g) alcohol h) acids & bases, pH >11, pH<3 i) metal ions, silver, copper (algal blooms) Indicator Organisms Impractical to analyze for all pathogens, so indicators are a “group” of organisms, like: - Fecal coliforms: exposure to fecal mammalian fecal matter Unfortunately for P & P industry: effluents often contain naturally occurring bacteria, Klebsiella Pneumonia, which registers as fecal coliforms. A more specific indicator, Escherichia Coli (E. coli) is being adapted in many places (including Michigan). Reliable so far…… Physical Disinfection Mechanisms 1. Damage to cell membrane 2. Alteration of cell membrane permeability - allows for potential escape of vital nutrients: N, P 3. Alteration of colloidal nature of protoplasm - e.g., coagulation of cell protein 4. Inhibition of enzyme activity 5. Alteration of organism DNA, RNA Desirable Properties of Disinfectants 1. Be effective under conditions normally found in wastewater: pH, temp 2. Non-toxic to humans at concentrations needed to disinfect 3. Reasonable cost 4. Safe to transport and apply 5. Concentration easily measured (dosage) 6. Maintain a residual (not shown in Table 12.1, 4th Ed.) Only one agent meets all of the above: chlorine (next choice probably ozone (O3) Some facts • Chlorine is found in the Earth itself and, as salt, in the seas which cover seven tenths of the planet. More facts • Worldwide, waterborne diseases kill over 25,000 people each day. Drinking water chlorination is one of the most widely used methods to safeguard and protect drinking water supplies. • It’s fundamental to the life of plants and animals. What’s it for? • Chlorine is also extensively used in the production of paper products, dyestuffs, textiles, petroleum products, medicines, antiseptics, insecticides, food, solvents, paints, plastics, many other consumer products, and disinfectants Comparison of Ozone and Chlorine 1. O3 more efficient: faster and smaller dosage 2. O3 more expensive 3. O3 has no residual 4. O3 has no taste or odor problems Chlorine Addition • Highly soluble, ~7,000 mg/L • Residuals desired (1 mg/L) not a problem • Shipped in large steel cylinders: certain risks associated Note: use of hypochlorite compounds is an alternative which provides exactly the same disinfectant at lower risk due to handling, but at higher cost. Chlorine Chemistry + Cl2 + H 2O ← → HOCl + H + Cl hydrolysis (fast and complete at pH >3) − HOCl ← → OCl + H ionization hypochlorous acid + hypochlorite ion HOCl + OCl = “free available chlorine” − not a disinfectant We could quit here if the wastestream was pure water…… However, nitrogen compounds get in the way by reacting with chlorine Factors Influencing Disinfection Efficacy and Microbial Inactivation • Microbe type: Resistance to chemical disinfectants: – Vegetative bacteria: Salmonella, coliforms, etc. – Enteric viruses: coliphages, HAV, SRSVs, etc. – Protozoan (oo)cysts, spores, helminth ova, etc. • Cryptosporidium parvum oocysts • Giardia lamblia cysts • Clostridium perfringens spores • Ascaris lumbricoides ova • Acid-fast bacteria: Mycobacterium spp. Least Most Distribution of Hypochlorous Acid and Hypochlorite in Water HOCl more effective (100:1) Chlorine Reactions with Ammonia HOCl + NH 3 ↔ H 2O + NH 2Cl (monochloramine) HOCl + NH 2Cl ↔ H 2O + NHCl2 HOCl + NHCl2 ↔ H 2O + NCl3 (dichloramine) (nitrogen trichloride) (no germicidal value, leaves as gas) Distribution = f(pH, temp., time, Cl2:NH3) Breakpoint Chlorination Nomenclature free chlorine (available & residual) = HOCl + OCl(gas) combined = NH2Cl + NHCl2 + NCl3 Relative effectiveness HOCl 1 OCl0.01 NH2Cl 0.005 NHCl2 0.04 Contact Time – Chick’s Law (1908) For a given concentration of disinfectant, the longer the contact time, the greater the kill. dN = −kN t dt Where: dNt/dt = rate of change in the concentration of organisms with time k = inactivation rate constant, T-1 Nt = number of organisms at time t t = time Note: departures from this law are common. Concentration of Disinfectant Depending on the type of chemical agent, it has been observed that, within limits, disinfection effectiveness is related to concentration. ' k =kC n Where: k = inactivation rate constant k’ = die-off constant C = concentration of disinfectant n = coefficient of dilution Note: n = 1 both the concentration and time are equally important n > 1 concentration is more important than time n < 1 time is more important than concentration