L-form - My Teacher Pages
advertisement

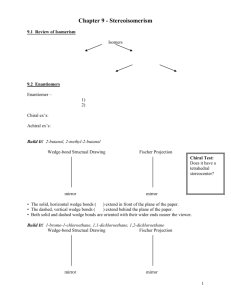
Food Chemistry Chapter 17 in Green / Damjii F.9: Texture Homework • Read F9– Texture pp. 488-490 • Do Qs 43-50 • on p 494 F.9.1: Explain the three different conventions used for naming the different enantiomeric forms. Types of Isomerism – II cont. • Stereoisomers – Enantiomers (aka Optical Isomers) • Requires a molecule with a chiral center – An atom (usu C) with four different groups attached to it – aka an asymmetric carbon atom • Two forms are mirror images of one another that cannot be superimposed on each other (like gloves) achiral chiral 24.2 Types of Isomerism – II cont. • Stereoisomers – Enantiomers • Common example are amino acids – only glycine does not have an enantiomeric form Types of Isomerism – II cont. • Stereoisomers – Enantiomers • Common example are amino acids – only glycine does not have an enantiomeric form - why? Types of Isomerism – II cont. Thalidomide • a drug once prescribed to counteract pregnancy-related morning sickness, is an effective sedative as the R enantiomer (left side), but the S enantiomer (right side) is a potent teratogen (it causes birth defects). Source - http://www.answers.com/topic/asymmetric-synthesis-1 Types of Isomerism – II cont. • Stereoisomers – Enantiomers • have different effects on polarised light • said to be optically active in a polarimeter • if a mixture has both enantiomers it is said to be a racemic mixture and is not optically active F.9.1: Explain the three different conventions used for naming the different enantiomeric forms. CONVENTION 1: – dextrorotary [ + or (d) ] – clockwise rotating enantiomer; positive specific rotation value – laevorotatory [ - or (l) ] – counter clockwise rotating enantiomer; negative specific rotation value Does differentiate between the 2 enantiomers… but does NOT indicate absolute configuration (spatial arrangement). F.9.1: Explain the three different conventions used for naming the different enantiomeric forms. CONVENTION 2: D, L (no relation to d and l ) – older convention… used mostly for sugars and amino acids – Most naturally occurring sugars are in the D-form ! (and taste sweet) – Most amino acids are in the L-form ! (and are tasteless) – Enantiomers that don’t occur naturally are typically NOT metabolized by our bodies. F.9.1: Explain the three different conventions used for naming the different enantiomeric forms. CONVENTION 2: D, L (no relation to d and l ) – For sugars… absolute configuration related to glyceraldehyde • • • • Locate chiral center Orient aldehyde group away from you If OH is on right, the sugar is DIf OH is on left, the sugar is L- – Most naturally occurring sugars are in the D-form ! (and taste sweet) F.9.1: Explain the three different conventions used for naming the different enantiomeric forms. CONVENTION 2: D, L (no relation to d and l ) – For amino acids… use “CORN” rule • • • Locate chiral center and orient C-H bond away from you If the groups COOH, R, NH2 are arranged clockwise around the chiral carbon the amino acid is the D-form If the groups COOH, R, NH2 are arranged counterclockwise around the chiral carbon the amino acid is the L-form – Most amino acids are in the L-form ! (and are tasteless) F.9.1: Explain the three different conventions used for naming the different enantiomeric forms. CONVENTION 3: R, S – Newer convention… used for most other compounds – aka CIP system – named after originators: Cahn, Ingold, and Prelog F.9.1: Explain the three different conventions used for naming the different enantiomeric forms. SYSTEM 3: R,S Locate chiral center Rank atoms bonded to the chiral carbon in order of increasing atomic number. (One through four, one being the group of highest priority… highest atomic number). H < C < N < O < F < Cl < Br If there are two or more atoms with same atomic number the second atoms are used to rank the substituents… then the third… etc. Orient the molecule so that the lowest ranking (4) substituent points away from you. If the other three substituents decrease in a clockwise direction, it is the R-enantiomer If the other three substituents decrease in a counterclockwise direction, it is the S-enantiomer F.9.1: Explain the three different conventions used for naming the different enantiomeric forms. Orient the molecule so that the lowest ranking substituent points away from you. If the other three substituents decrease in a clockwise direction, it is the Renantiomer If the other three substituents decrease in a counterclockwise direction, it is the S-enantiomer F.9.1: Explain the three different conventions used for naming the different enantiomeric forms. Orient the molecule so that the lowest ranking substituent points away from you. If the other three substituents decrease in a clockwise direction, it is the Renantiomer If the other three substituents decrease in a counterclockwise direction, it is the S-enantiomer F.9.1: Explain the three different conventions used for naming the different enantiomeric forms. (S)-(+)-lactic acid (left) and (R)-(–)-lactic acid (right) F.9.1: Explain the three different conventions used for naming the different enantiomeric forms. Enantiomers of citalopram (antidepressant). The top is (S)citalopram The bottom is (R)citalopram F.9.1: Explain the three different conventions used for naming the different enantiomeric forms. This molecule (on left side) needs to be reoriented so the C-H bond is facing away from us Enantiomers of mecoprop, 2-(4-chloro-2-methylphenoxy)propanoic acid – a herbicide F.9.2: Distinguish between the properties of the different enantiomeric forms of stereoisomers found in food. • Natural flavors tend to be pure enatiomers – biosynthesis is stereospecific • Synthetic flavors tend to be racemic mixtures Alpha-ionone (found in raspberries) Natural = R-alpha-ionone • Stereoisomers – Enantiomers • Usually have similar physical properties – except when they interact with other optically active substances – which happens often in the human body • Usually have similar chemical properties – except when they interact with other optically active substances – which happens often in the human body – general, only one enantiomer of a drug, agrochemical (herbicide, pesticide), flavoring agent, or other molecule (when asymmetric) has the desired biological effect, while the other enantiomer has very different effects or, at least, places a metabolic burden on the body. Types of Isomerism – II cont. amino acid asparagine - taste • one form tastes bitter, the other tastes sweet • each of the two enantiomers binds differently to chemoreceptors in the tongue. Source - http://www.answers.com/topic/asymmetric-synthesis-1 Carvone forms two mirror image forms or enantiomers: • • R-(–)-carvone smells like spearmint (happens to be laevorotary) S-(+)-carvone, smells like caraway seeds. – each of the two enantiomers binds differently to chemoreceptors in the nose Limone forms two mirror image forms or enantiomers: • R + (d) enantiomer - smells like orange • S – (l) enantiomer - smells like lemon (R)-(+)-(E)-alpha-ionone (S)-(-)-(E)-alpha-ionone – • • • • • woody • cedar wood like • fresh juicy greenish flavor(aroma) violet-like fruity raspberry-like flowery ( Yamamoto et al., 2009) ( Yamamoto et al., 2009)