BIORANSFORMATION
advertisement

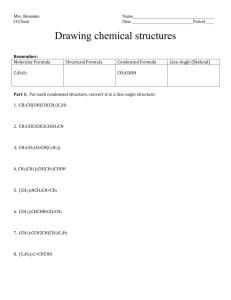
BIOTRANSFORMATION Prof. Dr. Basavaraj K. Nanjwade M. Pharm., Ph. D Department of Pharmaceutics KLE University’s College of Pharmacy BELGAUM – 590010, Karnataka, India Cell No: 0091 9742431000 E-mail: bknanjwade@yahoo.co.in 03-12-2010 KLECOP, Nipani 1 Contents:Introduction Phase I reaction Phase II reaction Factors affecting Biotransformation Reference 03-12-2010 KLECOP, Nipani 2 Introduction: Important terms: Detoxication (detoxification) Prodrugs Active Metabolite Reactive Metabolite Metabolism 03-12-2010 Xenobiotics KLECOP, Nipani 3 Definition: “Biotransformation of drug is defined as the conversion from one chemical form to another”. the term is used synonymously with metabolism. 03-12-2010 KLECOP, Nipani 4 Biotransformation leads to: • Pharmacologic Inactivation of Drug • Ex: Active Drug Salicylic Acid Inactive Drug Salicyluric Acid • Active Metabolite From An Inactive Drug • Ex. Inactive(Prodrug) • Aspirin Active Salicylic Acid •No Change in Pharmacologic Activity • Ex. 03-12-2010 Active Codeine Active Drug Morphine KLECOP, Nipani 5 Drug metabolizing organs • Liver is the heart of metabolism • Because of its relative richness of enzymes in large amount. • Schematic chart of metabolizing organ (decreasing order) • Liver > lungs > Kidney > Intestine > Placenta > Skin > Brain > Testes > Muscle > Spleen 03-12-2010 KLECOP, Nipani 6 Drug Metabolizing Enzymes Non Microsomal Microsomal 03-12-2010 KLECOP, Nipani 7 Microsomal Enzymes • Found predominately in the smooth Endoplasmic Reticulum of liver • Other areas: – Kidney – Lungs – Intestinal mucosa Non-microsomal enzymes • Found in the cytoplasm and mitochondria of hepatic cells • Other tissues including plasma 03-12-2010 KLECOP, Nipani 8 Non-microsomal enzymes Microsomal Enzymes • Non-synthetic/ Phase I reactions – Most oxidation and reduction – Some hydrolysis • Synthetic/ Phase II reactions – ONLY Glucuronide conjugation 03-12-2010 • Non-synthetic/ Phase I reactions – Most hydrolysis – Some oxidation and reduction • Synthetic/ Phase II reactions – ALL except Glucuronide conjugation KLECOP, Nipani 9 Microsomal Enzymes • Inducible – Drugs, diet, etc. Non-microsomal enzymes • Not inducible 03-12-2010 KLECOP, Nipani 10 Why Biotransformation? • Most drugs are excreted by the kidneys. • For renal excretion drugs should: – have small molecular mass – be polar in nature – not be fully ionised at body pH • Most drugs are complex and do not have these properties and thus have to be broken down to simpler products. • Drugs are lipophilic in nature. 03-12-2010 KLECOP, Nipani 11 • Strongly bound to plasma proteins. • This property also stops them from getting eliminated. • They have to be converted to simpler hydrophilic compounds so that they are eliminated and their action is terminated. When and How? • Biotransformation occur between absorption and elimination from kidneys. • Drugs administered orally can biotransform in the intestinal wall. 03-12-2010 KLECOP, Nipani 12 03-12-2010 KLECOP, Nipani 13 Metabolic reaction: Phase I reaction Phase II reaction Oxidation Conjugation Reduction Hydrolysis 03-12-2010 KLECOP, Nipani 14 Phase I: A polar functional group is either introduced or unmasked if already present on the otherwise lipid soluble Substrate, E.g. –OH, -COOH, -NH2 and –SH. Thus, phase I reactions are called as functionalization reactions. Phase I reactions are Non-synthetic in nature. The majority of Phase I metabolites are generated by a common hydroxylating enzyme system known as cytochrome P450. 03-12-2010 KLECOP, Nipani 15 03-12-2010 KLECOP, Nipani 16 Oxidative reaction: 1) Oxidation of aromatic carbon atoms 2) Oxidation of olefins (C=C bonds) 3) Oxidation of Benzylic, Allylic carbon atoms & carbon atoms alpha to carbonyl & imines 4) Oxidation of aliphatic carbon atoms 5) Oxidation of alicyclic carbon atoms 03-12-2010 KLECOP, Nipani 17 6) Oxidation of carbon-heteroatom systems: A. Carbon-Nitrogen system N- Dealkylation. Oxidative deamination N-Oxide formation N-Hydroxylation B. Carbon-Sulfur system S- Dealkylation Desulfuration S-oxidation C. Carbon-Oxygen systems(O- Dealkylation) 03-12-2010 KLECOP, Nipani 18 7) Oxidation of Alcohol, Carbonyle and Acid functios. 8) Miscellaneous oxidative reactions. Reductive reactions: 1) Reduction of Carbonyl functions.(aldehydes/ketones) 2) Reduction of alcohols and C=C bonds 3) Reduction of N-compounds (nitro,azo & N-oxide) 4) Miscellaneous Reductive reactions. 03-12-2010 KLECOP, Nipani 19 Hydrolytic reactions 1) Hydrolysis of Esters and Ethers 2) Hydrolysis of Amides. 3) Hydrolytic cleavage of non aromatic heterocycles 4) Hydrolytic Dehalogination 5) Miscellaneous hydrolytic reactions. 03-12-2010 KLECOP, Nipani 20 Oxidation of aromatic carbon atoms (aromatic hydroxylation): R OH Arenol (major) R R R OH H2O O Arene epoxide hydrase OH Arene oxide (highly reactive electrophile) Dihyrdrodiol GSH 5-epoxide transferase R OH SG Tissue toxicity in instances when glutathione is depleted. Glutathione conjugate (min.pro.) R OH OH Catechol(min. Pro.) E.g. Epoxides of Bromobenzene and Benzopyrene. 03-12-2010 KLECOP, Nipani 21 Oxidation of olefins (C=C bonds): Oxidation of non-aromatic C=C bonds is analogous to aromatic hydroxylation. i.e. it proceeds via formation of epoxides to yield 1,2dihydrodiols. OH HO O H2O N CONH2 Carbamazepine 03-12-2010 N CONH2 Carbamazepine-10,11 epoxide KLECOP, Nipani epoxide hydrase N CONH2 Trans-10,11 dihydroxy carbamazepine 22 Oxidation of Benzylic Carbon Atoms: Carbon atoms attached directly to the aromatic ring are hydroxylated to corresponding Carbinols. If the product is a primary carbinol, it is further oxidized to aldehydes and then to carboxylic acids, E.g.Tolbutamide A secondary Carbinol is converted to Ketone. CHO CH3 CH2OH SO2NHCONHC4H9 SO2NHCONHC4H9 Tolbutamide 03-12-2010 COOH Alcohol dehydrogenase Prmary carbinol Corresponding aldehyde KLECOP, Nipani Corresponding carboxylic acid. 23 Oxidation of Allylic carbon Atoms: Carbon atoms adjacent to Olefinic double bonds (are allylic carbon atoms) also undergo hydroxylation in a manner similar to Benzylic Carbons. E.g. Hydroxylation of Hexobarbital to 3`-hydroxy Hexobarbital. Allylic carbon atom OH 3' 2' H3C H3 C O O O HN O HN N N CH3 CH3 O O 3'-Hydroxy Hexobarbital Hexobarbital 03-12-2010 KLECOP, Nipani 24 Oxidation of Carbon Atoms Alpha to Carbonyls and Imines: Several Benzodiazepines contain a carbon atom (C-3) alpha to both Carbonyl (C=0) and imino (C=N) function which readily undergoes Hydroxylation. E.g. Diazepam O N N 3 OH N N IC IC 3-Hydroxy diazepam Diazepam 03-12-2010 KLECOP, Nipani 25 Oxidation of Aliphatic Carbon Atoms (Aliphatic Hydroxylation): Terminal hydroxylation of methyl group yields primary alcohols which undergoes further oxidation to aldehydes and then to carboxylic acid. H H3C C CH3 OH CH3 C H2 C H COOH C CH3 C H2 C H COOH Tertiary alcohol metabolite Ibuprofen 03-12-2010 H3C CH3 KLECOP, Nipani 26 Oxidation of Alicyclic Carbon Atoms (Alicyclic Hydroxylation): Cyclohexane (alicyclic) and piperidine (non-aromatic heterocyclic) rings are commonly found in a number of molecules. E.g. Acetohexamide and minoxidil respectively. Such rings are generally hydroxylated at C-3 or C-4 positions. H2N H2N N O N N N O 4' N N OH H2N H2N 4'-Hydroxy Minoxidil Minoxidil 03-12-2010 KLECOP, Nipani 27 Oxidation of Carbon-Heteroatom Systems: Biotransformation of C-N, C-0 and C-S system proceed in one of the two way: 1. Hydroxylation of carbon atom attached to the heteroatom and subsequent cleavage at carbonheteroatom bond. E.g. N-, O- and S- dealkylation, oxidative deamination and desulfuration. 2. Oxidation of the heteroatom itself. E.g. N- and S- oxidation. 03-12-2010 KLECOP, Nipani 28 Oxidation of Carbon-Nitrogen System: N-Dealkylation: Mechanism of N-dealkylation involve oxidation of αcarbon to generate an intermediate carbinolamine which rearranges by cleavage of C-N bond to yield the N dealkylated product and the corresponding carbonyl of the alkyl group. OH H O N C H 03-12-2010 N C NH C + H Carbinolamine Intermediate KLECOP, Nipani N-Dealkylated metabolite Carbonyl 29 A tertiary nitrogen attached to different alkyl groups undergoes dealkylation by removal of smaller alkyl group first. Example: Secondary aliphatic amine E.g. Methamphetamine. Tertiary aliphatic amine E.g. imipramine Tertiary alicyclic amine E.g. hexobarbital Amides E.g. Diazepam 03-12-2010 KLECOP, Nipani 30 N-Hydroxylation:- Converse to basic compounds that form N-oxide, Nhydroxy formation is usually displayed by non-basic nitrogen atoms such as amide Nitrogen. E.g. Lidocaine CH3 CH3 O N H C O C2H5 C H2 N N C H2 N OH C2H5 C2H5 CH3 CH3 N- Hydroxy Lidocaine Lidocaine 03-12-2010 C C2H5 KLECOP, Nipani 31 Oxidation of Carbon-Sulfur Systems: S-Dealkylation: The mechanism of S-Dealkylation of thioethers is analogous to N-dealkylation .IT proceed via α-carbon hydroxylation. The C-S bond cleavage results in formation of a thiol and a carbonyl product. E.g. 6-Methyl mercaptopurine. SCH2OH SCH3 N N N N N N N N H N H 6-Methyl Mercaptopurine 03-12-2010 SH Hydroxylated intermediate KLECOP, Nipani N N + HCHO N H 6-Mercaptopuri9ne 32 Desulfuration: This reaction also involves cleavage of carbon-sulfur bond (C=S). The product is the one with C=0 bond. Such a desulfuration reaction is commonly observed in thioamides such as thiopental. Thiopental KLECOP, Nipani Pentobarbital 33 S-Oxidation: Apart from S-dealkylation, thioethers can also undergo Soxidation reaction to yield sulfoxides which may be further oxidized to sulfones several phenothiazines. E.g. Chlorpromazine undergo S-oxidation. 03-12-2010 KLECOP, Nipani 34 Oxidation of Carbon-Oxygen Systems: O-Dealkylation: This reaction is also similar to N-Dealkylation and proceeds by α-carbon hydroxylation to form an unstable hemiacetal or hemiketal intermediate. Which spontaneously undergoes C-0 bond cleavage to form alcohol and a carbonyl moiety. H OH R-O-CH2R' Ether 03-12-2010 R-O-CH-R' Hemiacetal R-OH Alcohol KLECOP, Nipani + O=C-R' Aldehyde/ketone 35 Oxidation of Alcohol, Carbonyl and Carboxylic Acid In case of ethanol, Oxidation to acetaldehyde is reversible and further oxidation of the latter to acetic acid is very rapid since Acetaldehyde is highly toxic and should not accumulate in body. Aldehyde alcohol CH3CHO CH3CH2OH dehydrogenase Ethanol 03-12-2010 Acetaldehyde KLECOP, Nipani CH3COOH dehydrogenase Aceticacid 36 Miscellaneous oxidative reactions: Oxidative aromatization /dehydrogenation: E.g. Metabolic aromatization of drugs is nifedipine. COOH COOCH3 NO2 NO2 CH3 N NH CH3 COOCH3 CH3 COOCH3 Nitedipine 03-12-2010 CH2OH Pyridine metabolite KLECOP, Nipani 37 Oxidative Dehalogenation: This reaction is common with halogen containing drugs such as chloroform. Dehalogenation of this drug yields phosgene which may results in electrophiles capable of covalent binding to tissue. Cl Cl oxi. Cl H Chloroform 03-12-2010 O Cl Cl Covalent binding to tissues. -HCL Phosgene KLECOP, Nipani 38 Reductive reaction: Bioreductions are also capable of generating polar functional group such as hydroxy and amino which can undergo further biotransformation or conjugation. Reduction of carbonyls: Aliphatic aldehydes : E.g. Chloral hydrate Cl3C-CHOH2O Cl3C-CH2OH Chloral hydrate 03-12-2010 Trichloroethanol KLECOP, Nipani 39 Aliphatic ketones: E.g. Methadone. O OH C 2 H5 C 2 H5 H2 C H C N CH3 H2 C CH3 H3 C H3 C Methadone Aromatic Ketone: N CH3 CH3 Methadol E.g. Acetophenone. OH O C H CH3 CH3 Methyl phenyl carbinol Acetophenone 03-12-2010 H C KLECOP, Nipani 40 Reduction of alcohols and C=C: These two reductions are considered together because the groups are interconvertible by simple addition or loss of a water molecule. Before an alcohol is reduced it is dehydrated to C=C bond. Example – bencyclane . (antispasmodic) O (CH2)3 N(CH3)2 Bencyclane 03-12-2010 OH Bencyclanol KLECOP, Nipani Benzylidine cycloheptane 41 Reduction of N-compounds: Reduction of nitro groups proceeds via formation of nitro so and hydroxyl amine intermediates to yield amines. RNO2 R-N=O R-NHOH RNH2 Nitro Nitroso Hydroxylamine Amine For E.g. Reduction of Nitrazepam. N O2N H2 N Nitrazepam 03-12-2010 O H N O H N N 7-Amino metabolite. KLECOP, Nipani 42 Reduction of azo compounds yield primary amines via formation of hydrazo intermediate which undergo cleavage at N-N bond. R-N=N-R' RNH2 R-NH-NH-R' Azo E.g. Prontosil. Hydrazo N N Prontosil NH2R' Amines NH2 NH2 H2N + SO2NH2 H2N NH2 + H2N SO2NH2 1,2,4-Triamino benzene sulfanilamide It is reduced to active Sulfanilamide. 03-12-2010 KLECOP, Nipani 43 Miscellaneous reductive reactions: Reductive Dehalogenation: This reaction involves replacement of halogen attached to the carbon with the H-atom. E.g. Halothane. Br CF3 H CF3-CH3 CF3-COOH 1,1,1- Trifluroethane Trifluroacetic acid Cl Halothane Reduction of sulfur containing functional groups: C2H5 S N C2H5 Dsulfiram 03-12-2010 S S S N C2H5 C2H5 C2H5 E.g. Disulfuram S N SH C2H5 Diethyldithiocarbamic acid KLECOP, Nipani 44 Hydrolytic reactions: 1. The reaction does not involve change in the state of oxidation of substrate. 2. The reaction results in a large chemical chain in the substrate brought about by loss of relatively large fragments of the molecule. Hydrolysis of esters and ethers: Esters on hydrolyisis yield alcohol and carboxylic acid. The reaction is catalyzed by esterases. O R-C-OR' 03-12-2010 O R-C-OH KLECOP, Nipani + R'-OH 45 Organic acid esters: Esters with a large acidic group : E.g. Clofibrate COOC 2H5 Cl O CH3 COOH Cl O CH3 CH3 + CH3 Clofibrate C 2H5OH Free acid metan Esters with a large acidic and alcoholic group: E.g. Succinylcholine H2C COOCH2CH2-N(CH3)2 Pseudo C COOCH2CH2-N(CH3)2 CholineH2 sterase Suceinylcholine 03-12-2010 CH2COOH CH2COOH succinic acid KLECOP, Nipani + HOCH2CH2N(CH3)2 2 choline 46 Inorganic acid esters: Phosphates: E.g. Stilbestrol diphosphate C2 H5 OH C2 H5 OH O P O O P O C2 H5 OH CH3 H3C E.g. Isopropyl methanesulfonate CH3 O H3C O Isopropyl methnesulfonate 03-12-2010 2 H3PO4 stilbestrol O S CH3 H + C2 H5 OH Stilbestrol diphosphate Sulfates: OH HO OH H isopropanol KLECOP, Nipani O + HO S CH3 O methanesulfonic acid 47 Hydrolysis of amides: The reactions catalyzed by amides, involves C-N cleavage to yield carboxylic acid and amine. O O R-C-NHR' R-C-OH + R'NH2 primary amide with aliphatic substituent on N-atom: E.g. Procainamide C2H5 O H2N N C C N H H2 H2 C2H5 Procanamide 03-12-2010 C2H5 O + H2N H2N C C N C2H5 H2 H2 OH PABA KLECOP, Nipani 48 Secondary amide with aromatic Substituent on N-atom: E.g. Lidocaine CH3 CH3 O N H CH3 C 2H5 NH2 C N H2 C 2H5 + C 2H5 HOOC C N C 2H5 H2 CH3 Lidocaine Tertiary amide: 2,6 Xylidine N, N- Diethylglycine E.g. Carbamazepine N N CONH2 H Carbamazepine Iminostilbene 03-12-2010 KLECOP, Nipani 49 Hydrolytic cleavage of non aromatic heterocyclics: Four – membered lactams: O C H2 E.g. Penicillins O H N S N C H2 CH3 CH3 O CH3 N CH3 O Penicillin G COOH Penicinoic acid metabolite Five – member lactams: N E.g. Succinimides O Phensuximide O COOH NH2 CH3 03-12-2010 S HO COOH O H N Phenyl succinamic acid KLECOP, Nipani 50 Hydrolytic dehalogenation: Chlorine atoms attached to aliphatic carbons are dehalogenated easily. E.g. Dichloro diphenyl trichloro ethane H Cl Cl H -HCL Cl Cl CCl3 CCl2 DDT DDE Miscellaneous hydrolytic reactions: Include hydration of epoxides and arene oxides, hydrolysis of Sulfonylureas, Carbamates, Hydroxamates and alpha Glucuronide and sulfate conjugates 03-12-2010 KLECOP, Nipani 51 Phase 2 Reactions Synthetic Conjugation 03-12-2010 KLECOP, Nipani 52 Phase II • Phase II - combines functional group of compound with endogenous substance E.g. Glucuronicacid, Sulfuric acid, Amino Acid, Acetyl. Products usually very hydrophilic The final compounds have a larger molecular weight. 03-12-2010 KLECOP, Nipani 53 Enzymes • • • • Glucuronosyl Transferases Sulfotransferases (ST) Acetyltransferase Methylases 03-12-2010 KLECOP, Nipani 54 How We Get To Phase 2 • Most of the drugs do not become polar upon phase 1 reactions. • The Body is left with a plan to further metabolize the Drugs • Goal of Phase 2 : Make substances more soluble that couldn’t be done in the Phase 1 reactions. 03-12-2010 KLECOP, Nipani 55 Synthetic Reactions / Phase II • These reactions usually involves covalent attachments of small polar endogenous molecules such as Glucoronic acid, Sulfate, Glycine to either unchanged drugs or Phase I product having suitable functional groups as COOH,-OH,-NH2,- SH. • Thus is called as Conjugation reactions. • Since the product formed is having high molecular weight so called as synthetic reactions. • The product formed is hydrophilic in nature with total loss of pharmacologic activity so called as a true detoxification reaction 03-12-2010 KLECOP, Nipani 56 Phase II • • • • • • • • Glucuronide Conjugation Methylation Acetylation Sulfate Conjugation Conjugation With Alpha Amino Acids Glutathione Conjugation Glycine Conjugation Cyanide Conjugation 03-12-2010 KLECOP, Nipani 57 • Very important Synthetic reactions carried out by Uredine Di Phosphate Glucuronosyl Transferase. • Hydroxyl and Carboxylic acid groups are easily combined with Glucuronic acid. 03-12-2010 KLECOP, Nipani 58 Glucuronide formation occurs in 2 steps:- 1. Synthesis of an activated coenzyme uridine-5’- diphospho -alphaD- Glucuronic acid (UDPGA) from UDP- glucose (UDPG). -D-Glucose-1-phosphate + UTP Pyrophosphorylase UDPG + Ppi UDPG - Dehydrogenase UDPG +2NAD + H2O 03-12-2010 UDPGA +2NADH + 2H+ KLECOP, Nipani 59 2. Transfer of the glucuronyl moiety from UDPGA to the substrate RXH in presence of enzyme UDPUDP-Glucuronyl transferase glucuronyl transferase to form the conjugate. UDPGA + RXH Where, X = O, COO, NH or S 03-12-2010 RX Glucuronic Acid +UDP KLECOP, Nipani 60 COOH COOH COOH Benzoic acid OH OH UDP OH OH OH UDPGA OH Benzoic Acid Glucuronide Benzoic acid Ex. Chloramphenicol, Morphine, Salicylic Acid, Paracetamol 03-12-2010 KLECOP, Nipani 61 • Common, minor pathway. • Methyltransferases – -CH3 transfer to O, N, S, C 03-12-2010 KLECOP, Nipani 62 1. Synthesis of an activated coenzyme S- adenosyl methionine(SAM), the donor of methyl group, from Lmethionine and ATP. L – Methionine + ATP Methionine Adenosyl Transferase SAM + PPi + Pi 2. Transfer of the methyl group from SAM to the substrate in presence of nonmicrosomal enzyme methyl transferase. RXH + SAM Methyl Transferase RX-CH3 + S – Adenosyl Homocysteine Ex. Morphine, Nicotine, Histamine 03-12-2010 KLECOP, Nipani 63 Major route of biotransformation for aromatic amines, hydrazine. Generally decreases water solubility Enzyme: - N- Acetyltransferase (NAT) R – NH2 03-12-2010 R – NH – COCH3 KLECOP, Nipani 64 COOH COOH OH OH CH3COSCOA NH 2 Paraaminosalicyclic Acid Acetyl Co enzyme NHCOCH3 NH 2 N- Acetylated PAS Ex. Histamine, PAS, PABA 03-12-2010 KLECOP, Nipani 65 • Sulfotransferases are widely-distributed enzymes • Cofactor is 3’-phosphoadenosine-5’-phosphosulfate (PAPS) • Produce highly water-soluble sulfate esters, eliminated in urine, bile • R – OH 03-12-2010 R – O – SO3 KLECOP, Nipani 66 1. Synthesis of an activated coenzyme 3’-phosphoadenosine-5’phosphosulfate (PAPS) which acts as a donor of sulfate to the substrate. This also occurs in two steps- an initial interaction between the sulfate and the adenosine triphosphate (ATP) to yield adenosine-5’-phosphosulfate (APS) followed by activation of latter to PAPS. ATP Sulfurylase/Mg++ ATP + SO42- APS + ATP 03-12-2010 APS + Ppi APS Phosphokinase/Mg++ PAPS + ADP KLECOP, Nipani 67 2. Transfer of sulfate group from PAPS to the substrate RXH in presence of enzyme Sulfotransferase and subsequent liberation of 3’- phosphoadenosine-5’-phosphate(PAP). PAPS + RxH Sulfotransferase Rx-SO3 + PAP X= O,NH Examples of compounds undergoing sulfation are: Phenol Paracetamol , Salbutamol Alcohols Aliphatics C-1 to C-5 Arylamines Aniline 03-12-2010 KLECOP, Nipani 68 • Alternative to glucuronidation • Two principle pathways – -COOH group of substrate conjugated with -NH2 of Glycine, Serine, Glutamine, requiring CoA activation • E.g. conjugation of benzoic acid with Glycine to form Hippuric acid – Aromatic -NH2 or NHOH conjugated with -COOH of Serine, Proline, requiring ATP activation 03-12-2010 KLECOP, Nipani 69 1. Activation of carboxylic acid drug substrate with ATP and coenzyme A (CoA) to form an acyl CoA intermediate. Thus, the reaction is a contrast of glucuronidation and sulfation where the donor coenzyme is activated and not the substrate. Acetyl Synthetase RCOOH + ATP RCOAMP + H2O + Ppi Acyl CoA Transferase RCOAMP + CoA-SH RCSCoA + AMP 2. Acylation of the alpha- amino acid by the acyl CoA in presence of enzyme N-acyl transferase. RCSCoA + NH2-R’-COOH 03-12-2010 N-Acetyl transferase KLECOP, Nipani RCONH-R’COOH + CoA- SH 70 • Glutathione-S-transferase catalyzes conjugation with glutathione • Glutathione is tripeptide of Glycine, Cysteine, Glutamic acid 03-12-2010 KLECOP, Nipani 71 Glutathione-S-transferase γ-Glutamyl transferase 03-12-2010 KLECOP, Nipani 72 Glycine Cysteinyl glycinase N- acetylase Mercapturic Acid Derivative 03-12-2010 KLECOP, Nipani 73 Glycine Conjugation Salicylates and other drugs having carboxylic acid group are conjugated with Glycine. Not a major pathway of metabolism Cyanide Conjugation Conjugation of cyanide ion involves transfer of sulfur atom from thiosulfate to the cyanide ion in presence of enzyme rhodanese to form inactive thiocyanate. 2- S2O3 + CN 03-12-2010 - rhodanese KLECOP, Nipani - 2- SCN + SO3 74 Biotransformation-Conclusion • Change the Xenobiotics to a form that can be eliminated from the body • Change the Xenobiotics to a less biologically active form • Bioactivation to more toxic forms can also occur • Synthetic Phase II reactions are carried out by other enzymes. 03-12-2010 KLECOP, Nipani 75 Factor affection of Biotransformation of drug: The Therapeutic efficacy, Toxicity and Biological half life of drug depends on metabolic rate and the factor that influence metabolic rate are: 1) Physicochemical property of drug. 2) chemical factors: a. Induction of drug metabolizing enzyme. b. Inhibition of drug metabolizing enzyme c. Environmental chemicals. 3) Biological factors. A. Species differences. B. Strain differences. C. Sex differences. 03-12-2010 KLECOP, Nipani 76 D. Age. E. Diet. F. Altered pharmacologic factors: i Pregnancy. ii Hormonal imbalance. iii Disease state. G. Temporal factors: I Circadian rhythm. 03-12-2010 KLECOP, Nipani 77 References • Biopharmaceutics & Pharmacokinetics by D.M. Brahmankar, S. B. Jaswal, Vallabh Prakashan, Pg111-158. • Biopharmaceutics & Pharmacokinetics by Milo Gibaldi, 4th edition, Pg.no. 203. • Text book of Biopharmaceutics & pharmacokinetics, Dr.Sobha Rani R. Hiremath, Prism Books Pvt Ltd, Bangalore, 2000 Pg.no. 157-166. • www.google.com • www.pharmacology.com 03-12-2010 KLECOP, Nipani 78 Cell No: 0091 9742431000 E-mail: bknanjwade@yahoo.co.in 03-12-2010 KLECOP, Nipani 79