Cell Transport
advertisement
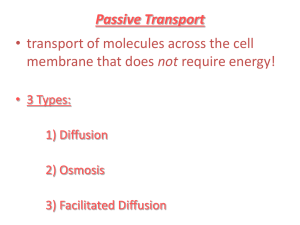
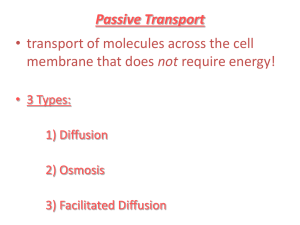
CH. 3 ~ CELLS Cell Membrane 1. Surrounds ALL cells 2. Has a “Phospholipid bilayer” which makes it “flexible and “fluid” Cell Membrane “Phospholipid bilayer” - Phosphate ‘head’ (polar- hydrophillic) - 2 Lipid ‘tails’ (nonpolar- hydrophobic) Cell Membrane 3. Semi-permeable – only allows certain substances to pass through 4. Contains proteins- some used for identification and some for transport (that help to pass materials through). Pics-Alpha/Beta Proteins- Text- p112 With a Hydrophobic Middle… how does Water get through? Aquaporins Osmosis: Passive movement of water across a membrane Two Ways: Diffusion and bulk flow (aquaporins) • The membrane possesses integral proteins; water transport integral proteins are called aquaporins. Serves as a water-filled pipe across the membrane.Dehydr..ADH/post pit…add aquaporins-Kidney/retain H O 2 Transport of materials into and out of the cell • Passive Transport – movement of molecules that does not require energy. – Usually from high concentration to lower concentration ex. Diffusion, Osmosis, Facilitated diffusion • Active Transport – movement of molecules that requires energy. – Usually from low concentration to high concentration (against the concentration gradient) ex. Pumps, endocytosis, exocytosis Review of Terms: • Equilibrium – when 2 given areas have the same concentration of molecules. • Concentration Gradient – the difference in concentration of molecules in 2 given areas. • Defines HOW molecules will move, if going WITH the concentration gradient (from high to low = passive transport) or AGAINST it (from low to high = requires energy = active transport Passive Transport: 1. Diffusion – movement of molecules from an area of high concentration to an area of lower concentration. Demos: Dye in Water Sugar Cube Passive Transport: 2. Osmosis – diffusion of water Demo: Raisin in Water 3. Facilitated diffusion – passive transport of materials across a cell membrane using protein channels or carriers. (aka “Transport Proteins” Increases the Rate of diffusion Facilitated Diffusion (TEXT: p 120- Example: Transport of Glucose) Demo: Golf Ball: Bind, Change Shape, Release…Both In & Out Protein Channels Carrier Proteins Facilitated Diffusion Increases the rate of diffusion • Similar to simple diffusion in the sense that it is diffusion (across a membrane) from a high concentration to a lower concentration. • However, this time the rate of diffusion is greatly accelerated by the action of membrane proteins that act as carrier molecules and aid in diffusion. http://www.northland.cc.mn.us/biology/Biology1111/animations/transport1.html P/S-Notepage Pictures of Passive and Active Transport Using a Protein Channel Energy Added Using a Protein Pump Active Transport: Pumps • Type of active transport • Move molecules from an area of LOW concentration to an area of HIGH concentration • One example: the Na-K Pump (Sodium-Potassium) • Requires Energy ACTIVE: Na-K Pump (See diagram- back of notes….Ex: Basketball Pump) *ONE Pump: does 450:300 Sodium OUT:Potassium IN every SECOND Na+ binds, ATP > ADP K+ released, ready for Na+ Shape change to original, release of K+ ATP > ADP Protein changes shape Na+ (3) released from cell, K+ binds K+ binds (2), releases another P Active: Endocytosis and Exocytosis http://www.northland.cc.mn.us/biology/Biology1111/animations/transport1.html ACTIVE Endocytosis • Intake of material (food) using the cell membrane • Cell membrane surrounds material and encloses it – forming a vesicle/vacuole. • Phagocytosis “cell eating” (WBC) • Pinocytosis “cell drinking” White Blood Cell (WBC) engulfing bacteria Amoeba Eating ACTIVE Exocytosis • Removal of material (waste) from a cell. • Golgi Apparatus “packages” the material into a vesicle and sends it to the cell membrane • The vesicle fuses with the membrane • The material is deposited outside the cell Exocytosis Electron Micrograph of Exocytosis • This figure was taken from Alberts et al, Molecular Biology of the Cell, Garland Publishing Third Edition, 1994 PASSIVE ACTIVE NO ENERGY ENERGY • Diffusion of perfume, food coloring • Osmosis • Facilitated diffusion – Protein channels – Carrier proteins • Exocytosis – GA packages and secretes wastes • Endocytosis – Membrane surrounds and engulfs food, etc. (Amoeba) • Pumps – Sodium-Potassium There are 3 types of osmotic solutions *Solute, Solvent, Solution Osmosis, Equilibrium/No Net Flow • Isotonic - equilibrium • Hypertonic – there is a greater concentration of solute (ie, salt, the ‘stuff’) in the solution than in the cell • Hypotonic – there is a greater concentration of solute (ie, salt, the ‘stuff’) in the cell then in the fluid around the cell LESS ? Solute outside MORE Solute outside EQUAL Solute outside Whenever solutes (dissolves substances) are added to water, they decrease the number of free water molecules; more solute molecules, fewer free water molecules Osmosis in Red Blood Cells Comparison of Plant and Animal Cells in Osmotic Solutions What is the major differences between what happens in plant and animal cells when placed in a hypotonic solution? Diffusion and Osmosis Khan Academy 18.59 Onion Cell PLASMOLYSIS Secondary Cell Wall From the adjoining cell- DO NOT DRAW on diagram of ONE CELL Primary Cell Wall The ONE line to have in your drawing Cell Mambrane After Plasmolysis DIALYSIS DEMO • The small molecules of water and iodine moved from an area of high concentration, outside the cell, to an area of low concentration inside the cell in order to try to reach equilibrium. The large molecules of starch were not able to diffuse and reach equilibrium because they were too large to pass through the semi-permeable membrane. Potential to move…. Pressure to keep out…. Hypoosmotic Hyperosmotic OP vs WP Semi-Permeable Water Molecule Low OP,High WP Hydration Shell High OP,Low WP Hydration Shells: Water molecules surrounding solutes A -3 B -7 Plants & water potential • The combined effects of 1.) solute concentration 2.) physical pressure (cell wall) can be measured as Water Potential • = psi • is measured in megapascals (MPa) • 1 Mpa = 10 atmospheres of pressure Calculating Solute potential • Need solute concentration • Use the equation S = - iCRT i = # particles molecule makes in water C = Molar concentration R = pressure constant 0.0831 liter bar mole oK T = temperature in degrees Kelvin = 273 + oC (25) Solve for water potential (literal equation) • Knowing solute potential, water potential can be calculated by inserting values into the water potential equation. = P + S In an open container (or animal cell), P = 0 Diffusion and Osmosis Lab • Bozeman 7.46 Water Potential Explained- powerpoint