Document
advertisement

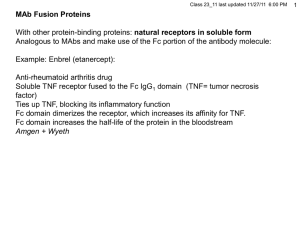
1 Protein glycosylation Adds another layerof structure and specificity to proteins Can enhance the function of a protein Can extend the lifetime of a protein Can help localize a protein within a cell Can act as a specific antigen 2 Two types of protein glycosylation N-acetyl group glucose galactose 3 1 1 2 3 Pentasaccharide common core 2 = = All shown Triantennary here, (also tetra-antennary) N-linked (to amide N of Asn in N-X-S or N-X-T) Carbohydrates attached to exterior loops or near termini Sia= sialic acid (see below) Diantennary With bisecting GlcNAc With fucosylated core Substantial in size Fucose Also O-linked, to ser or thr (hydroxyl on side chain); see below 4 Enlargement for display 5 anomeric carbon Fisher view Chair view Haworth view 6 11 10 7 6 5 89 3 1 24 7 Glucose } Gray = C White = H Red = O C1 C6 (-CH2OH) C5 Ring oxygen Alpha or beta? Polysaccharide formation 8 down H H or glycogen chain 9 Examples of O-linked oligosaccharides O-linked oligosaccharides usually consist of only a few carbohydrate residues, which are added one sugar at a time. 10 C4 glucose Examples of other hexoses C2 galactose mannose allose What’s different from glucose here? 11 Metabolic intermediate (Bacterial cell walls) (Insect exoskeleton) 12 Carbohydrate structure specific for: Cell type Physiological state No. of sites depends on 3-D structure of protein Structure at that site depends on the site E.g., transferrin, from different cell types : Cerebrospinal fluid (made in brain): diantennary asialoagalactofucosylated bisecting GlcNAc Blood (made in liver): diantennary NAcNeu (sialated= sialic acid) afucosylated Sialic acid structure: see next graphic 13 neuraminic acid – one of the sialic acids = : both terms are used, confusedly NAcNeu: Carboxyl (acid) Glycerol moiety Mannose framework Acetylated amino group deoxy 14 Glycosylation pattern affects signaling of proteins used therapeutically, for: Delivery of the soluble glycoprotein drug to the right cell receptor for activity Clearance rate Microheterogeneity: Lots of isoforms typically present Glycosylation does not seem to represent a bottleneck in high-producing cells: 0.1 mg/l (amplify) 200 mg/l = same pattern Insect cells (Baculovirus, high level transient expression for production): Too simple a pattern compared to human Mouse and hamster cells: similar to human Hamster: less heterogeneity 15 Genetic engineering of glycosylation to: Modify or enhance activity E.g.: Better binding to a receptor More specific binding Different binding, in theory Also: Antigenicity Clearance rate Decrease microheterogeneity (for clinical application) 16 Modifying glycosylation 1. Add or subtract sites to your favorite protein (cis) 1a. Subtract sites: Easy, change N or S or T to A by site-directed mutagenesis 1b. Add sites: Not so easy. Consensus N-X-S does not work, e.g.: Requires the insertion of a ~12 aa region encompassing a real N-glycosylation site (6 suffices for O-linked) Place on an end or on a loop (must know protein’s structure) Works 2. Change the general glycosylation phenotype of the host cell (trans) E.g., Pam Stanley: lectin-resistant mutants Modifying glycosylation 1. Add or subtract sites to your favorite protein (cis) 2. Change the general glycosylation phenotype of the host cell (trans) 2. Clone enzyme genes: Glycosyl transferases, mostly Also some synthetases (e.g., NAcNeu synthetase) Can be complex: e.g., 7 different fucosyl transferases (FTs), with different (overlapping) substrate specificities Simpler example: Hamster cells do only 2,3 sialylation. Humans do 2,6 as well, via a 2,6-sialyl transferase (ST) Experiment: Over-express cloned human 2,6 ST, along with a substrate protein; produce permanent transfectants of BHK cells (BHK = baby hamster kidney) Get both types of structures now, substantially (although not exactly the same ratio as in human cells). J Biol Chem, Vol. 273, Issue 47, 30985-30994, November 20, 1998 In Vivo Specificity of Human 1,3/4Fucosyltransferases III-VII in the Biosynthesis of LewisX and Sialyl LewisX Motifs on Complex-type N-Glycans. COEXPRESSION STUDIES FROM BHK-21 CELLS TOGETHER WITH HUMAN -TRACE PROTEIN Eckart Grabenhorst , Manfred Nimtz , Júlia Costa§, and Harald S. Conradt ¶ 17 18 Isolate mutant mammalian cell lines deficient in specific glycosylation enzymes Stanley: Isolation of multiply mutated glycosylation mutants by selecting lectin resistance Lectins = carbohydrate-binding proteins Plant lectins used mostly here (but occur widely in animals as well) Sequential selections, push - pull on resistance, sensitivity Resistance: enzyme deficiency failure to add the sugar need for lectin binding Sensitivity: failure to add a sugar produces greater exposure of underlying sugars A transferase-negative mutant better binding to the exposed sugar Showed power of selection, usefulness of complementation via cell hybridization Review: Nature Biotechnology 19, 913 - 917 (2001) , The bittersweet promise of glycobiology. Alan Dove Pam Stanley 19 Hybrid selection: • All lec-R mutants were: WGA (wheat germ agglutinin) resistant (various degrees) & pro- (required proline) • • • • Tester parent was single lec-R + GAT- (req’d glycine, adenine and thymidine) Select in medium lacking pro and GAT, and with +/- WGA Complementing hybrids will have regained sensitivity to WGA Mutants in the same gene will remain WGA resistant (non-complementation) • • Potential: build a production cell line with all glycosyltrasnferases, etc. mutated out. Could now be used as a tabla rasa (blank slate) introducing a series of enzymes to build custom tailored glyco-conjugates. Complicated though (order of addition, location in the Golgi, etc. ) Mostly not developed yet. 20 Umana, P., Jean-Mairet, J., Moudry, R., Amstutz, H., and Bailey, J.E. 1999. Engineered glycoforms of an antineuroblastoma IgG1 with optimized antibody-dependent cellular cytotoxic activity. Nat Biotechnol 17: 176-180. Target here (bisecting NAcG) (NAcG = N-acetyl-glucosamine here) Presence of the bisecting NAcG enhances binding of T-cell receptor to the Fc region of antibodies. Binding is needed for ADCC. Mouse and hamster cell lines used for commercial production lack the glycosyltransferase needed for bisecting NAcG addition A rat myeloma cell line does produce MAb with the bisecting NAcG. Hypothesis: Expression of the rat enzyme in a CHO cell line will add a bisecting NacG to the anti-neuroblastoma MAb produced by these cells. The modified MAb will be a better mediator of ADCC. Experiment: Clone the cDNA for this enzyme from the rat line and transfer it to CHO cells, driven by an inducible tet promoter. Check sugar structure of Mab (MS) and ADCC efficiency of the Mab (in vitro lysis). ADCC 21 TARGET CELL Genentech (Killer T-cell) Commercial MAb injected as a therapeutic T-cell surface receptor binds Fc region of antibody molecule (Fc gammaR) 22 Umana, P., Jean-Mairet, J., Moudry, R., Amstutz, H., and Bailey, J.E. 1999. Engineered glycoforms of an antineuroblastoma IgG1 with optimized antibody-dependent cellular cytotoxic activity. Nat Biotechnol 17: 176-180. Low tet, tet-off system, = higher production Neuroblastoma cells + NK T-cells + antibody Cytotoxicity Yet lower tet, tet-off system, = yet higher production No tet, tet-off system, = highest production non-optimal High tet, tet-off system, = basal production Anti-neuroblastoma anibody (ng/ml) 23 Protein Glycosylation Assigned: Naoko Yamane-Ohnuki, et al.. Establishment of FUT8 knockout Chinese hamster ovary cells: an ideal host cell line for producing completely defucosylated antibodies with enhanced antibody-dependent cellular cytotoxicity. Biotechnol Bioeng. 2004 Sep 5;87(5):614-22 Optional Update: Kanda Y, Yamane-Ohnuki N, Sakai N, Yamano K, Nakano R, Inoue M, Misaka H, Iida S, Wakitani M, Konno Y, Yano K, Shitara K, Hosoi S, Satoh M. Comparison of cell lines for stable production of fucose-negative antibodies with enhanced ADCC. Biotechnol Bioeng. 2006 Jul 5;94(4):680-8. Review: Grabenhorst, E., Schlenke, P., Pohl,., Nimtz, M., and Conradt, H.S. 1999. Genetic engineering of recombinant glycoproteins and the glycosylation pathway in mammalian host cells. Glycoconj J 16: 81-97. Background: Stanley, P. 1989. Chinese hamster ovary cell mutants with multiple glycosylation defects for production of glycoproteins with minimal carbohydrate heterogeneity. Mol Cell Biol 9: 377-383. 24 Biotechnol Bioeng. 2004 Sep 5;87(5):614-22 Hypothesis: Fucose interferes with binding of the T-cell’s Fcgamma3 receptor to the Fc region of an antibody molecule. Elimination of fucose from produced MAbs will increase ADCC. Create a mutant CHO cells (starting with amplifiable dhfr- cells) in which the fucose transferase (biosynthesis) genes have been knocked out. All mAbs produced in these mutant cells will be better at promoting ADCC Double knock-out strategy for FUT8 an alpha-1,6,fucosyl transferase 25 Little sequence data available for Chinese hamster Isolate CHO cDNA using mouse sequence data for primers Use CHO cDNA to isolate CHO genomic fragments from a commercial lambda library K.O. exon 1 translation start region Homology regions DT= diphtheria toxin gene, Kills if integrated via non-homologous recombination For hemizygote: Select for G418 resistance, Screen by PCR for homologous recomb. 108 cells 45,000 colonies 40 false recombinants (extension-duplications) + 1 true recombinant Step 2 for homozygote, select for Pur-resistance Lox sites 1.6X10870,000 screened 10 double KO homozygotes. Remove drug resis. genes by transient transfection with Cre Recombinase. Exon 1 suffers a 200 nt deletion Note: 10’s of thousands of PCRs performed to screen for homologous recomb., using 96-well plates 26 Double knockout evidence After Cre treatment Original KO’d genes have a 1.5 kb insertion (Southern blot) mRNA has 200 nt deletion (RT-PCR) 27 Use of a fluoresceinated lentil lectin (LCA) that binds fucose oligosaccharides to demonstrate lack of fucosylation in glycosylated proteins in the FUT8 -/- cells Control background fluorescence (FL-anti avidin) FUT8 +/+ FUT8 +/Surprising: CHO cells do not have excess fucosylation capacity FUT8 -/- Rituxan (retuximab, anti-CD20) produced in FUT -/- cells does not contain fucose (HPLC analysis) Digestion all the way to monosaccharides Missing d - g 28 Binding to CD20 membranes In ADCC, FUT8-/- anti-CD20 >> Rituxan 29 FUT8-/- anti CD20 = Rituxan Anti-CD20 from a partially FUT-deficient rat cell line Fc-Receptor protein binding assay Rat line Complement-mediated cell toxicity is the same for FUT8-/- and Rituxan FUT-/-’s Rituxan = commercial product, 98% fucosylated 30 Very laborious, but apparently a big payoff. Better selection?: Why not use the fluorescent LCA to select for the FUT8 KO’s along with G418 resistance (double sequential selection)? 31 Hans Henning von Horsten et al., Glycobiology vol. 20 no. 12 pp. 1607–1618, 2010 Production of non-fucosylated antibodies by co-expression of heterologous GDP-6-deoxy-D-lyxo-4-hexulose reductase (RMD) Clone bacterial RMD cDNA Construct mam. expn vector Transfect into CHO cells making Herceptin (anti EGF receptor) Deflects intermediate in fuciose biosynthetic path 32 Select for G418 resistance, screen for lack of fucose. WT CHO cells One of 3 clones: No fucose in transfectant glycoproteins Also absent by MS 33 Binding assay to Fc receptor (ELISA-type assay) About10-fold more effective 3 transfectants WT Antibody concentration (ng/ml) ELISA = Enzyme-linked immunosorbent assay 34 ADCC lysis assay vs. a HER2+ breast carcinoma cell line % lysis About10-fold more effective 3 transfectants WT Concentration of anitbody