Immunogenicity: The key issue for multisource biologics
advertisement

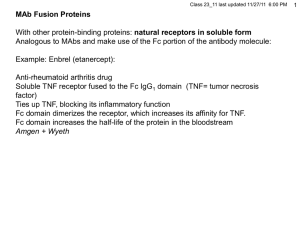
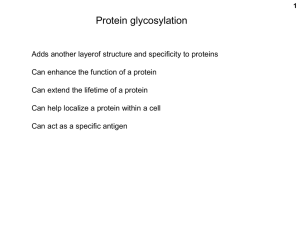
Immunogenicity and glycosylation: The key issues for biosimilars Huub Schellekens Utrecht University Immunogenicity and biotech comparability Current analytical methods cannot fully predict biological properties The immune system can detect alterations in products missed by analytical methods Immunogenicity of biopharmaceuticals may have serious clinical consequences History of the medical use proteins Proteins of animal origin (e.g. equine antisera, porcine/bovine insulin): foreign proteins Human derived proteins (e.g.growth hormone, factor VIII): no immune tolerance Recombinant human proteins(e.g.insulin, interferons, GM-CSF): ?? Most biopharmaceuticals induce antibodies Two mechanisms Reaction to neo-antigens Breakdown of immune tolerance Types of immune reaction against biopharmaceuticals Breaking of self-tolerance Type of product Human homologues Characteristics of antibody production Slow, after long treatment, binding antibodies, disappear after treatment Cause Mainly impurities and aggregates Factors influencing immunogenicity Structural properties Sequence variation Glycosylation Other factors Assays Contaminants and impurities Formulation Downstream processing Route of application Dose and length of treatment Patient characteristics Unknown factors Consequences of antibodies Loss of efficay Insulin Enhancement of efficacy Streptokinase Staphylokinase Growth hormone ADA Salmon calcitonin Factor VIII Neutralization of native protein Interferon alpha 2 MDGF Interferon beta EPO IL-2 GnRH TNFR55/IgG1 General immune effects Denileukin diftitox Allergy HCG Anaphylaxis GM-CSF/IL3 Serum sickness, etc Pure red cell aplasia associated with EPO treatment Data from Nicole Casadevall Bone Marrow Smear Normal Bone Marrow PRCA Bone Marrow Pure red cell aplasia associated with anti-EPO antibodies Nicole Casadevall - 1996 PRCA case with natural antibodies - 2002 13 cases with antibodies associated with epoetin treatment Why was Eprex implicated? High association between Eprex and PRCA Geographic distribution Association with formulation change Time course of individual PRCA cases 1997 1998 1999 2000 2001 2002 2003 J F M A M J J A S O N D J F M A M J J A S O N D J F M A M J J A S O N D J F M A M J J A S O N D J F M A M J J A S O N D J F M A M J J A S O N D J Since Dec 93 Since Feb 93 t Epo-refractory anemia (diagnosis) Pure Red Cell Aplasia (diagnosis) Epoetin alfa SC Eprex Epoetin alfa IV Eprex Epoetin beta SC NeoRecormon Darbepoetin Since Dec 95 Product formulation – Recent concern over use of HSA in Europe because of potential transmission of infectious viruses or BSE prions – In 1998, HSA was replaced with polysorbate 80 in prefilled syringes of Eprex® distributed ex-US Main stabilizers used in the epoetin formulations Epogen®/Procrit® Eprex® (US) (pre 1998) Eprex® (post 1998) NeoRecormon® (1990 launch) HSA Polysorbate 80 Polysorbate 20 Glycine Glycine HSA Complex of 5 other amino acids Calcium chloride Urea Factors potentially contributing to the immunogenicity of Eprex® Formation of micelles associated with Epo (Hermeling et al, 2003) Silicon droplets in the prefilled syringes Leachates from rubber stoppers Mishandling What is the role of micelles? Very unstable No biological data Does not explain epidemiological data Silicon as adjuvant Lot of confusion data in the literature Silicon is inert The leachate theory No biological rationale – Adjuvants do not break B cell tolerance No experimental data showing breaking tolerance Does not explain epidemiological data and pathogenesis Mishandling Mishandling with a slightly less stable product may explain all features of PRCA – Biological rationale – Fits with data concerning other product – Fits the pathogenesis – Fits with the epidemiological data Prediction of immunogenicity Purity of the product Epitope analysis Reaction with patient sera Animal experiments • Convential animals (relative immunogenicity) • Non-human primates • Immune tolerant transgenic mice Daily i.p. 5 ug Avonex Transgenic immune-tolerant 5 ug Avonex Wildtype (C57Bl/6) t=0 t=7 t=14 t=21 0.50 0.25 0.75 A 415nm - A 490nm A 415nm - A 490nm 0.75 t=0 t=7 t=14 t=21 0.50 0.25 0.00 0.00 1 2 3 Mouse 4 5 1 2 3 Mouse 4 5 40 ug Betaseron s.c. 2x/week Wildtype (C57Bl/6) t=0 t=7 t=14 t=21 0.75 0.50 0.25 0.00 1 -0.25 2 3 4 5 Mouse 5 ug Betaseron daily i.p. Transgenic immune-tolerant 1.00 A 415nm - A 490nm A 415nm - A 490nm 1.00 t=0 t=7 t=14 t=21 0.75 0.50 0.25 0.00 1 -0.25 2 3 Mouse 4 Reducing immunogenicity Optimizing production,purification and formulation Changing sequence (streptokinase, staphylokinase) Pegylation (ADA) Glycosylation Also an important issue in the biosimilar discussion Glycosylation of biosimilar epoetins can be expected to be different What types of glycosylation are there? What is the biological significance of glycosylation O-linked glycosylation O-linked to specific serine or threonine but consensus sequence not identified Apparently defined by secondary structural elements like β turn. Start with the attachment of a single monosaccharide normally Nacetylgalactoseamine Then extended by glycosyltransferases N-linked glycosylation N-linked to Asn-X-Ser/Thr Most consensus sequences non-glycosylated. Depends on secondary structures. Glycosylation before folding Starts with binding of DTP-oligosaccharide: 2 GlcNAc, 9 mannose and 3 glucose molecules. Trimming by removing glucoses and mannoses and possible adding of GlcNAc The functions of the glycocomponent Protein folding Protein trafficking Protein targeting Ligand recognition Ligand binding Biological activity Stability Pharmacokinetics Immunogenicity Glycosylation of epoetin 40% sugar Three linked N-glycosylation sites at Asn 24, 38 and 83 One O-linked site at serine 126 Heterogeneity caused by variation in core structures and sialic acid Removal of N-glycosylation sites has no effect on in vitro activity, but greatly reduces the in vivo activity Half life in rodents IV 5-6h but < 2 min if desialylated Adding N-glycosylation sites increases half-life. Single O-linked side chain removal has little effect ? Aberrant glycosylation biosimilar epoetins Retracrit: > glycoforms without O-glycans. < N-glycolyl and 0-acetyl neuraminic acid Epoetin alpha Hexal: > high mannose Epoetin lacking O-linked glycosylation About 20% lesser activity Delorme at al. Biochemistry 1992 Explanation for the lower activity of Retracrit? Conclusion The clinical consequences of immunogenicity may be severe Only clinical trials decisive to reveal immunogenicity The main difference between biosimilars is glycosylation Clinical consequences of differences in glycosylation unknown What are the unanswered questions? – – – – What is biosimilar? Naming Label Safety monitoring Sensitivity Background data Standardization – Price Counterfeits