Adrenal glands
advertisement

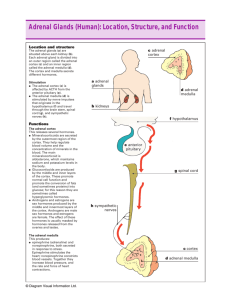
Adrenal glands and hormones Adrenal glands The adrenals are orange-colored glands that sit on top of the kidneys near the spine, just underneath the last rib and extending down about an inch. The right adrenal is shaped something like a pyramid, whereas the left is shaped more like a half moon. Each adrenal gland is composed of two endocrine components: a medulla (inner part) that constitutes 20% of the gland and a cortex (outer part) that constitutes the remaining 80%. The cortex consists of three zones. The medulla and each of the zones in the cortex each produce different hormones that serve a variety of functions in your body. Adrenal Glands The Adrenal Cortex The adrenal cortex is divided into three zones which each secrete different hormones that carry out specific functions throughout your body. 1. Zone of glomerulosa Cortex 1. Zone of glomerulosa 2 3 Medulla Aldosterone is secreted from this zone which is the major hormone controlling the sodium and potassium levels, and thus fluid balance, within your bloodstream, cells and interstitial fluids. It is also called mineralocorticoids. Cortex The Adrenal Cortex 1. Zone of glomerulosa 2. Zone of fasciculata 2 Coritsol (hydrocortisone) is produced, affects glucose, amino acid and fat metabolism, which is called glucocorticoids. Cells of this zone are arranged into fascicles separated by venous sinuses. 3. Zone of reticularis 3 Medulla Dehydroepiandrosterone (DHEA)-precursor for androgen is synthesized. This zona manufactures an ancillary portion of sex hormones for each sex and also produces male hormones in women and female hormones in men to keep the effects of the dominant sex hormones in balance . Adrenals CORTEX Zona Glomerulosa Mineralocorticoids (Aldosterone) Na+, K+ and water homeostasis Zona Fasciculata Glucocorticoids (Cortisol) Glucose homeostasis and many others Zona Reticularis Sex steroids (androgens) Medulla: “Catecholamines” Epinephrine, Norepinephrine, dopamine Synthesis of adrenocorticosteroids All steroid hormones have in common the 17-carbon cyclopentaoperhydrophenanthrene nucleus. Additional carbons can be added at positions 10 and 13 or as a side chain attached to C17. Synthesis of adrenocorticosteroids Steroid hormones and their precursors and metabolites differ in 1. number and type of substituted groups, 2. number and location of double bonds, 3. stereochemical configuration. 21 18 18 17 19 3 Synthesis of adrenocorticosteroids 1. Uptake of cholesterol by the adrenal cortex is mediated by the LDL receptor. With long-term stimulation of the adrenal cortex by ACTH, the number of LDL receptors increases. Much of the cholesterol in the adrenal is esterified and stored in cytoplasmic lipid droplets. Synthesis of adrenocorticosteroids 2. Upon stimulation of the adrenal by ACTH or cAMP, an esterase is activated, and the free cholesterol formed is transported into the mitochondria. Synthesis of adrenocorticosteroids 3. In the mitochondria, a cytochrome P450 side chain cleavage enzyme (P450SCC) converts cholesterol to pregnenolone. Synthesis of adrenocorticosteroids 4. Pregnenolone may be converted by dehydrogenase/isomerase to progesterone or else by P450c17 (17-α-hydroxylase) to 17αhydroxypregnenolone. Progesterone can also be converted to 17αhydroxyprogesterone by P450c17. 17-α-hydroxylase dehydrogenase/isomerase 17-α-hydroxylase Synthesis of adrenocorticosteroids 5. After the synthesis of progesterone and 17-hydroxyprogesterone, P450c21(21-hydroxylase) can hydroxylate these steroids at the 21 position, resulting in 11-deoxycorticosterone and 11-deoxycortisol, respectively. 21-hydroxylase 21-hydroxylase 6. The final step in the synthesis of adrenal mineralocorticoids and glucocorticoids is mediated by P450c11 (11-β-hydroxylase), which also mediates the final steps in the synthesis of aldosterone from deoxycorticosterone. 11 11-β-hydroxylase 11-β-hydroxylase Synthesis of adrenocorticosteroids 7. P450c17 has two activities, that of a 17α-hydroxylase and that of a C17,20 lyase capable of breaking up the C-17,20 carbon bond of 17αhydroxypregnenolone or 17α-hydroxyprogesterone, yielding dehydroepiandrosterone (DHEA) or androstenedione, respectively. Synthesis of adrenocorticosteroids 8. 17-hydroxysteroid dehydrogenase convert androstenedione to testosterone. P450aro mediates the aromatisation of androgens to estrogens in the gonads. In peripheral target tissues, testosterone can further be converted to 5αdihydrotestosterone by 5α-reductase. Biosynthesis Of human steroid Hormones Some autosomal recessive mutations in biosynthetic enzymes responsible for converting cholesterol to androgens generally lead to partial male-to-female sex reversal. CAH (congenital adrenal hyperplasia): For P450c21 is deficiency, cortisol synthesis decreases, leading to overproduction of ACTH. When this occurs adrenal steroid synthesis is stimulated and 17hydroxyprogesterone is converted to androstenedione and further to testosterone, leading to severe virilization of the female fetus. This disorder is known as CAH which disrupts the synthesis of all adrenal and gonadal steroids. Affected genetic males are born with normal female external genitalia. Biochemical actions of adrenocorticosteroids A. Mineralocorticoids: aldosterone It promotes Na+ reabsorption at the distal convoluted tubules of kidney. Na+ retention is accompanied by corresponding excretion of K+,H+ and NH4+ ions. Biochemical actions of adrenocorticosteroids B. Glucocorticoids: Cortisol Biochemical actions of adrenocorticosteroids B. Glucocorticoids: Cortisol 1. Effects on glucose metabolism: They promote gluconeogenesis. They work in tandem with insulin from the pancreas to maintain blood glucose levels in the proper balance. 2. Effects on lipid metabolism: They increase lipolysis in adipose tissue and reduce synthesis of TAG. 3. Effects on protein and nucleic acid metabolism: They promote transcription and protein synthesis in liver. They also cause catabolic effects in extrahepatic tissues results in enhanced degradation of protein. 4. Effects on water and electrolyte metabolism: Deficiency of them cause increased production of ADH which can decrease glomerular filtration rate causing water retention in the body. 5. Effects on immune system: Cortisol suppress the immune response directly and indirectly by affecting most cells that participate in immune reactions and inflammatory reactions. It is powerful anti-inflammatory even when secreted at normal levels. It also reduces the rate at which lymphocytes multiply and accelerates their programmed cell death to further protect the body from this overreaction. This is one of the reasons why strong corticosteroids (prednisone, prednisolone, etc.) are used with all diseases involving inflammatory processes, including autoimmune diseases. 6. Effects on cardiovascular system: Cortisol could control the contraction of the walls of the mid-sized arteries in increasing blood pressure, but this hypertensive effect is moderated by calcium and magnesium. It also directly affects the heart by regulating sodium and potassium in the heart cells and increasing the strength of contraction of the heart muscle. 7. Effects on central nervous system: The changes of behavior, mood, excitability and even the electrical activity of neurons in the brain frequently occur in cases of excess and deficient cortisol levels. Many signs and symptoms of adrenal fatigue involve moodiness, decreased tolerance, decreased clarity of thought and decreased memory. These occur because the brain is affected by both too little and too much cortisol. Stress Adrenal glands are the anti-stress glands of the body. There are four major categories of stress: 1. Physical stress: such as overwork, lack of sleep, athletic overtraining. 2.Chemical stress: environmental pollutants, allergies to foods, diets high in refined carbohydrates, endocrine gland imbalances. 3. Thermal stress: over-heating or over-chilling of the body 4. Emotional and mental stress Stress: During stress cortisol must simultaneously provide more blood glucose, mobilize fats and proteins for a back-up supply of glucose, modify immune reactions, heartbeat, blood pressure, brain alertness and nervous system responsiveness. If cortisol level cannot rise in response to these needs, maintaining your body under stress is nearly impossible. Hypothalamopituitary adrenal (HPA) axis Immune system: altered Stress Circadian rhythm Hypothalamus CRH Anterior Pituitary Gland (-) Posterior Pituitary Gland ACTH Glucocorticoids, Adrenals Catecholamines, etc.. Kidney Muscle: Net loss of amino Acids (glucose) Liver: Deamination of proteins into amino acids, gluconeogenesis (glucose) Fat Cells: Free fatty acid mobilization Heart rate: Increased (Figure 9-40) Fasting People have considerable difficulty when on a prolonged fasting. They will always rationalize the problems encountered on a fasting as being due to the body detoxifying. During a fasting, the body will call on the adrenals to produce glucocorticoids to maintain blood glucose level which is adequate for normal level of activity. The glucocorticoids can elevate blood glucose by breaking down protein into carbohydrates through the process of gluconeogenesis. Regulation of glucocorticoids The Secretion of glucocorticoids from the adrenal cortex is regulated by negative feedback involving the CRH secretion by the hypothalamus. CRH then acts on the anterior pituitary to stimulate ACTH secretion, which then stimulates the adrenal cortex into cortisol secretion. About 70% of blood cortisol is bound to a carrier protein called corticosteroidbinding globulin. Another 15% is bound to albumin, the remaining 15% exists free in solution. ※ The Hypothalamus/Pituitary/Adrenal (HPA) Axis The HPA axis or HPA system, a negative feedback system, is one of the most important elements of homeostasis, the process that maintains a steady internal biochemical and physiological balance in your body. The HPA Axis adjusts cortisol level according to the needs of the body, under normal and stressed conditions, via ACTH. ACTH is secreted from the pituitary gland in response to orders form the hypothalamus and travels in the bloodstream to the adrenal cortex. Addison's Disease: Primary Chronic Adrenocortical Insufficiency People who suffer from adrenal fatigue almost always have some form of irregular blood sugar pattern, of which hypoglycemia is the most common. When your adrenals are fatigued, their cortisol output is diminished and you have lower levels of circulating blood cortisol, your liver has a more difficult time converting glycogen into glucose. Cushing's syndrome (hyperadrenocorticism or hypercorticism) is a endocrine disorder caused by high levels of cortisol (hypercortisolism) in the blood. This can be caused by taking glucocorticoid drugs, or by tumors that produce cortisol or ACTH. Cortex The Adrenal Medulla The functional unit of the adrenal medulla is the chromaffin cell, which functions as a neuroendocrine cell. In response to stimulation, chromaffin cells secrete the hormones epinephrine (adrenaline) and norepinephrine (noradrenalin) directly into the blood. 1 2 3 Medulla The medulla is involved in extreme stress and, within this context, epinephrine and norepinephrine both work with cortisol from the adrenal cortex. Epinephrine and norepinephrine are important mainly in crisis situations. Catecholamines Biosynthesis 1. Tyrosine is precursor for the synthesis of catecholamines. 2. The catecholamine are produced in response to fight, fright and flight (3F). These include emergencies like shock, cold, fatigue, emotional condition like anger. Biochemical function of catecholamine 1. Effect on carbohydrate metabolism: Both of them can increase glycogenolysis and gluconeogenesis and decrease glycogenesis. ①Catecholamine promote the release of glucose from liver and decrees its utilization by muscle; ②Epinepherine inhibits insulin secretion but promote glucagon secretion. 2. Effect on lipid metabolism: Both of them enhance the breakdown of TAG in adipose tissue. This cause increase in the free fatty acid in the circulation which are effectively utilized by the heart and muscle as fuel source. 3. Effect on physiological function: Cateccholamines increase cardiac output, blood pressure and oxygen consumption. They cause smooth muscle relaxation in bronchi, GIT and blood vessels supplying skeletal muscle. phaeochromocytoma (PCC) or pheochromocytoma: a neuroendocrine tumor of the medulla of the adrenal glands (originating in the chromaffin cells), or extraadrenal chromaffin tissue that failed to involute after birth and secretes excessive amounts of catecholamines, usually adrenaline (epinephrine) if in the adrenal gland and not extra-adrenal, and noradrenaline (norepinephrine). 17% of adrenal cases are bilateral (suggesting hereditary disease) 18.4% in children (also suggesting hereditary disease) 5% are extra-adrenal (located in any orthosympathetic tissue): of these 9% are in the abdomen and 1% are located elsewhere. Some extra-adrenal phaeochromocytomas are probably actually paragangliomas, but the distinction is only possible after surgical resection. 11.1% malignant, but this rises to 30% for extra-adrenal cases 26% are hereditary 3% recur after being resected 14% of affected individuals do not have arterial hypertension Here's a look at the extra-adrenal sites of pheochromocytomas 1. Within the sympathetic nerve chain along the spinal cord (orange spots) 2. Overlying the distal aorta (the main artery from the heart) (green spots) 3. Within the ureter (collecting system from the kidney (yellow spot) 4. Within the urinary bladder (blue spot) 5. Remember, 90% are in the adrenal glands (red spots on the kidneys)