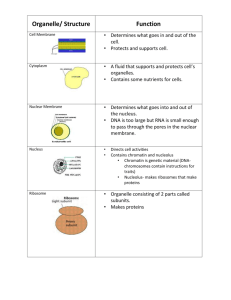
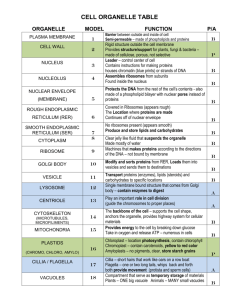
Endoplasmic reticulum Lect. 8
advertisement

The endoplasmic reticulum (ER) in the cells of eukaryotic organisms is an interconnected network of flattened, membrane-enclosed sacs or tubes known as cisternae (latin “reservoir for liquid”) Endoplasmic means “within the cytoplasm,” and reticulum latin “little net” The membranes of the ER are continuous with the outer membrane of the nuclear envelope. Membrane of ER separates the lumen of ER from cytoplasm but continuous with perinuclear space. ER occurs in most eukaryotic cells except RBCs and spermatoza. There are two types of ER, rough endoplasmic (RER) and smooth endoplasmic reticulum (SER). The outer (cytosolic) face of the RER is studded with ribosomes the sites of protein synthesis. RER is prominent in hepatocytes where active protein synthesis occurs The SER lacks ribosomes and functions in lipid metabolism, carbohydrate metabolism, and detoxification. SER is abundant in mammalian liver and gonad cells. The ER were first seen in 1945 by Keith R, Porter, Albert Claude, Brody Meskers and Ernest F. Fullam reticulum 1 Nucleus 2 Nuclear pore 3 Rough endoplasmic reticulum (RER) 4 Smooth endoplasmic reticulum (SER) 5 Ribosomes on the rough ER 6 Proteins that are transported 7 Transport vesicle 8 golgi apparatus 9 Cis face of the Golgi apparatus 10 Trans face of the Golgi apparatus 11 Cisternae of the Golgi apparatus 3-D MODEL OF ENDOPLASMIC RETICULUM The ER is a membranous network of cisternae (sac-like structures) held together by the cytoskeleton. The phospholipid membrane encloses a space, the cisternal space (lumen), which is continuous with the perinuclear space but separated from the cytosol. The functions of the ER are the synthesis and export of proteins and membrane lipids. The quantity of RER and SER in a cell can slowly interchange, depending on the changing metabolic activities of the cell. Transformation can include embedding of new proteins in membrane as well as structural changes. Changes in protein content may occur without noticeable structural changes The surface of RER is studded with ribosomes giving it a "rough" appearance. The binding site of the ribosome to RER is the transcolon. Ribosomes are constantly being bound and released from the RER membrane. A ribosome only binds to the RER once a specific protein-nucleic acid complex forms in the cytosol. This special complex forms when a free ribosome begins translating the mRNA of a protein destined for the secretary pathway. The first 5-3 AAs polymerized encode a signal peptide, a molecular message that is recognized and bound by a signal recognition particle (SRP). Translation pauses and the ribosome complex binds to the RER transcolon where translation continues with the nascent protein forming into the RER lumen. The protein is processed in the ER lumen by an enzyme (signal peptidase), which removes the signal peptide. Ribosomes at this point may be released back into the cytosol; however, non-translating ribosomes stay associated with transcolons. There is no continuous membrane between the ER and golgi apparatus, membrane-bound vesicles shuttle proteins between these two compartments Vesicles are surrounded by coating proteins COPI and COPII. COPII directs vesicles to golgi and COPI brings them back to RER. The RER works in concert with the golgi complex to target new proteins in vesicles to their proper destinations. A second method of transport out of the ER involves areas called membrane contact sites, where the membranes of the ER and other organelles are held closely together, allowing the transfer of lipids and other small molecules The RER manufactures lysosomal enzymes with a mannose-6phosphate marker added in the cis-Golgi network RER manufactures secreted proteins (glycoproteins) glycosylation occurs in lumen of RER. RER is involved in synthesis of signal peptides, integral membrane proteins, Rab proteins the key in targeting the membranes and SNAP proteins involved in fusion event and glycosylation. RER makes its own phospholipids and enzymes of ER membrane. RER is component of endomembrane system. SER is membranous network without ribosomes in the cytoplasm near the periphery of cells. SER is involved in several metabolic processes. It synthesizes lipids, oils, phospholipids and steroids. Cells which secrete these products, such as those in the testes, ovaries, and skin oil glands have large amount of SER. SER also carries out the metabolism of carbohydrates SER detoxify drugs and poison in liver cells by adding hydroxyl groups to drug molecules making them soluble and easier to flush out from body. Drugs like phenobarbital and barbiturates metabolized by SER by proliferation to increase the detoxification enzymes. It may increase the tolerance towards other drugs i.e. Barbiturates abuse decrease the effectiveness of certain antibiotics. SER involves in the attachment of receptors on cell membrane proteins, and steroid metabolism. In muscle cells, it regulates calcium ion concentration. SER contains the enzyme glucose-6-phosphatase, which converts Glu-6-phosphate to glucose, a step in glucogenesis. SER produces sex hormones in vertebrates and steroid hormones that are secreted by adrenal glands. The sarcoplasmic reticulum (SR), from the Greek sarx, ("flesh"), is smooth ER found in smooth and striated muscles. The only structural difference between SR and SER is the medley of proteins both bound to their membranes and drifting within their lumens. This fundamental difference indicates their functions: The SER synthesizes molecules, while SR stores and pumps calcium ions. The SR contains large stores of calcium, which releases when the muscle cell is stimulated. It plays a major role in excitation contraction coupling. ER is involved in the folding of protein molecules by several ER proteins in cisternae and transport them in vesicles to Golgi apparatus. Only properly folded proteins are transported from the RER to the Golgi apparatus. Disturbances in redox regulation, calcium regulation, glucose deprivation, and viral infection or the over-expression of proteins can lead to ER stress, a state in which the folding of proteins slows, leading to an increase in unfolded proteins. Protein transport Secretory proteins, glycoproteins, are moved across the ER membrane. Proteins that are transported by the ER called a signal sequence. The N-terminus of a polypeptide chain contains a few amino acids that work as signal sequence which are removed when the polypeptide reaches its destination. Proteins are packed into transport vesicles and moved along cytoskeleton toward their destination. The ER is also part of a protein sorting pathway. It is, the transportation system of the eukaryotic cell. The majority of its resident proteins are retained within it through a retention motif. This motif is composed of four amino acids at the end of the protein sequence.