Organic 2 PPT
advertisement

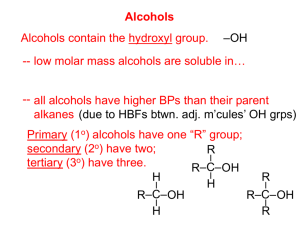
“Functional Groups” Functional Groups Most organic chemistry involves substituents –often contain O, N, S, or P –also called “functional groups”- they are the chemically functional part of the molecule, and are the nonhydrocarbon part Functional Groups Functional group - a specific arrangement of atoms in an organic compound, that is capable of characteristic chemical reactions. –What is the best way to classify organic compounds? By their functional groups. Functional Groups The symbol “R” is used to represent any carbon chains or rings - alkyl groups Halogen Substituents Halocarbons - class of organic compounds containing covalently bonded fluorine, chlorine, bromine, or iodine – General formula: R-X (X = halogen) Naming? Name parent as normal, add the halogen as a substituent (or prefix) Halogen Substituents The more highly halogenated the compound is, the higher the b.p. Few halocarbons found in nature –but, readily prepared and used –halothane and also the hydrofluorocarbons Substitution Reactions Organic reactions often much slower than inorganic reactions – must break strong covalent bond – trying to find new catalysts to use Substitution - an atom (or group of atoms) replaces another atom or group of atoms Substitution Reactions A halogen (shown as “X”) can replace a hydrogen to make a halocarbon: R-H + X2 R-X + HX Sunlight is often a sufficient catalyst: UV light CH4 + Cl2 → CH3Cl + HCl Substitution Reactions Treating benzene with a halogen? Halogens on carbon chains are readily displaced by hydroxide ions (OH1-) to make an alcohol + a salt: R-X + OH1- R-OH + X1CH3-Cl + NaOH CH3-OH + NaCl Methanol + sodium chloride Substitution Reactions CH3-I + KOH CH3-OH + KI Iodomethane Methanol CH3CH2Br + NaOH CH3CH2OH + NaBr Bromoethane Ethanol Alcohols and Ethers Alcohols Alcohols - a class of organic compounds with an -OH group – The -OH functional group in alcohols is called a “hydroxyl” group; thus R-OH is the formula How is this different from the hydroxide ion? (covalent bonding with the carbon- not ionic with a metal like bases) Alcohols Aliphatic alcohols classified into categories according to the number of R groups attached to the carbon with the hydroxyl –1 R group: primary alcohol –2 R groups: secondary alcohol –3 R groups: tertiary alcohol Alcohols Both IUPAC and common names For IUPAC: –drop the -e ending of the parent alkane name; add ending of -ol, number the position of -OH –parent is the longest chain that contains the carbon with the hydroxyl attached. Alcohols The hydroxyl is given the lowest position number Alcohols containing 2, 3, and 4 of the -OH substituents are named diols, triols, and tetrols respectively Alcohols Common names: –similar to halocarbons, meaning name the alkyl group, then followed by the word alcohol –One carbon alcohol = methyl alcohol Alcohols More than one -OH substituents are called glycols (ethylene glycol?) Phenols - compounds in which a hydroxyl group is attached directly to an aromatic ring. Cresol is the common name of o, m, and p isomers of methylphenol Properties of Alcohols Much like water, alcohols are capable of hydrogen bonding between molecules –this means they will boil at a higher temp. than alkanes and halocarbons with a comparable number of atoms Properties of Alcohols Alcohols are derivates of water; the -OH comes from water, and thus are somewhat soluble Alcohols of up to 4 carbons are soluble in water in all proportions; more than 4 carbons are usually less soluble, because the longer carbon chain is more nonpolar Properties of Alcohols Many aliphatic alcohols used in laboratories, clinics, and industry –Isopropyl alcohol (2-propanol) is rubbing alcohol; used as antiseptic, and a base for perfume, creams, lotions, and other cosmetics Ethylene glycol (1,2-ethanediol) commonly sold as “antifreeze” Properties of Alcohols Glycerol (1,2,3-propanetriol) used as a moistening agent in cosmetics, foods, and drugs; also a component of fats and oils Ethyl alcohol (ethanol) used in the intoxicating beverages; also an important industrial solvent Properties of Alcohols Denatured alcohol- means it has been made poisonous by the addition of other chemicals, often methyl alcohol (methanol, or wood alcohol). As little as 10 mL of methanol has been known to cause permanent blindness, and 30 ml has resulted in death! Addition Reactions The carbon-carbon single bond is not easy to break In double bonded alkenes, it is easier to break a bond Addition reaction- substance is added at the double or triple bond location, after it is broken Addition Reactions Addition of water to an alkene is a hydration reaction - usually occurs with heat and an acid (such as HCl or H2SO4 acting as a catalyst) Note the formation of ethanol from ethene + water Addition Reactions If a halogen is added in an addition reaction, the result is a halocarbon that is disubstituted – The addition of bromine is often used as a test for saturation Addition of a hydrogen halide? called monosubstituted halocarbon Addition Reactions Addition of hydrogen to produce an alkane is a hydrogenation reaction, which usually involves a catalyst such as Pt or Pd –common application is the manufacture of margarine from unsaturated vegetable oils (making them solid from a liquid) Addition Reactions The hydrogenation of a double bond is a reduction reaction, which in one sense is defined as the “gain of H” ethene is “reduced” to ethane; cyclohexene is “reduced” to cyclohexane Ethers A class of organic compounds in which oxygen is bonded to 2 carbon groups: R-O-R is formula Naming? The two R groups are alphabetized, and followed by ether Two R groups the same? Use the prefix di- Ethers Diethyl ether is the one commonly called just “ether” –was the first reliable general anesthetic –dangerous- highly flammable, also causes nausea ethers are fairly soluble in water Alcohol used for fuel in the future? Carbonyl Compounds Aldehydes and Ketones Review: –alcohol has an oxygen bonded to a carbon group and a hydrogen –ether has an oxygen bonded to two carbon groups An oxygen can also be bonded to a single carbon by a double bond Aldehydes and Ketones The C=O group is called the “carbonyl group” –it is the functional group in both aldehydes and ketones Aldehydes - carbonyl group always joined to at least one hydrogen (meaning it is always on the end!) Aldehydes and Ketones Ketones - the carbon of the carbonyl group is joined to two other carbons (meaning it is never on the end) Aldehydes and Ketones Naming? –Aldehydes: identify longest chain containing the carbonyl group, then the -e ending replaced by -al, such as methanal, ethanal, etc. –Ketones: longest chain w/carbonyl, then new ending of -one; number it? propanone, 2-pentanone, 3-pentanone Aldehydes and Ketones Neither can form intermolecular hydrogen bonds, thus a much lower b.p. than corresponding alcohols wide variety have been isolated from plants and animals; possible fragrant odor or taste; many common names Aldehydes and Ketones Benzaldehyde Cinnamaldehyde Vanillin Methanal (the common name is: formaldehyde) –40% in water is formalin, a preservative Aldehydes and Ketones Propanone (common: acetone) is a good solvent; miscible with water in all proportions why is it a good substance used in nail-polish removers? (a powerful solvent-able to dissolve both polar & nonpolar) The Carboxylic Acids… Also have a carbonyl group (C=O), but is also attached to a hydroxyl group (-OH) = “carboxyl” group general formula: R-COOH –weak acids (ionize slightly) Named by replacing -e with -oic and followed by the word acid methanoic acid; ethanoic acid Carboxylic Acids Abundant and widely distributed in nature, many having a Greek or Latin word describing their origin –acetic acid (ethanoic acid) from acetum, meaning vinegar –many that were isolated from fats are called fatty acids The Esters… General formula: RCOOR Derivatives of the carboxylic acids, in which the -OH from the carboxyl group is replaced by an -OR from an alcohol: carboxylic acid + alcohol ester + water many esters have pleasant, fruity odors- banana, pineapple, perfumes Esters Although polar, they do not form hydrogen bonds (reason: there is no hydrogen bonded to a highly electronegative atom!) –thus, much lower b.p. than the hydrogen-bonded carboxylic acids they came from Esters Can be prepared from a carboxylic acid and an alcohol; usually a trace of mineral acid added as catalyst (because acids are dehydrating agents) Esters Naming? It has 2 words: –1st: alkyl attached to single bonded oxygen from alcohol –2nd: take the acid name, remove the -ic acid, add -ate Oxidation- Reduction Reactions All of the previous classes of organic compounds are related by oxidation and reduction reactions What is oxidation-reduction? –Oxidation: the gain of oxygen, loss of hydrogen, or loss of e-1 –Reduction: the loss of oxygen, gain of hydrogen, or gain of e-1 Oxidation- Reduction Reactions Oxidation and reduction reactions (sometimes called redox) are coupled- one does not occur without the other The number of Oxygen and Hydrogen attached to Carbon indicates the degree of oxidation Oxidation- Reduction Reactions The fewer the # of H on a C-C bond, the more oxidized the bond –Thus, a triple bond is more oxidized than a double bond and a single bond An alkane is oxidized (loss of H) to an alkene, and then to an alkyne Oxidation- Reduction Reactions Loss of hydrogen is called a dehydrogenation reaction –may require strong heating and a catalyst Oxidation- Reduction Reactions Methane can be oxidized in steps to carbon dioxide methane methanol methanal methanoic acid CO2 the more reduced (more H) a carbon compound, the more energy it can release upon oxidation Oxidation- Reduction Reactions Alcohols can also be oxidized into other products “Dr. Al K. Hall Mr. Al D. Hyde” Preparing aldehydes from a primary alcohol is a problem, because they are then easily oxidized to carboxylic acids Oxidation- Reduction Reactions Benedict’s test and Fehling’s test are commonly used for aldehyde detection – Polymerization Addition Polymers Polymers are giant molecules, not small like the ones studied earlier in this chapter –examples are plastics Polymer- large molecule formed by the covalent bonding of smaller molecules called monomers Polymers from Monomers Addition Polymers An addition polymer forms when unsaturated monomers react to form a polymer –ethene will form polyethylene, –polyethylene is easy to clean, chemically resistant- milk bottles, plastic wrap, refrigerator dishes High Density Polyethylene Addition Polymers Polypropylene is a stiffer polymer, used in utensils and containers Polystyrene is formed from styrene (phenylethene), and is a poor heat conductor (styrofoam ® Dow Chemical) –molded coffee cups and picnic coolers, insulates homes Polyvinyl chloride (PVC) used for pipes in plumbing Addition Polymers Polytetrafluoroethene (PTFE, or teflon ® DuPont) is very resistant to heat and chemical corrosion –found on nonstick cookware; coating on bearings and bushings used in chemical reactors Condensation Polymers Condensation polymers are formed by the head-to-tail joining of monomer units –usually accompanied by the loss of water from the reacting monomers, and forming water as a product Condensation Polymers Ex: polyethylene terephthalate (PET) –Dacron (® DuPont), Fortrel (® Wellman), Polyesters: permanent press clothing, tire cords –Sheets of polyester called Mylar (® DuPont), used as magnetic tape in tape recorders and computers, as well as balloons –Nylon: carpet, fishing line, hosiery Condensation Polymers Examples: –aromatic rings form Nomex (® DuPont), which is a poor electrical conductor; makes parts for electrical fixtures; flame resistant clothing for race car drivers; flame resistant building materials –Kevlar (® DuPont): strong and flame resistant Plastic container code system. CODE MATERIAL PERCENT OF TOTAL Polyethylene Terephthalate (PET) 20-30 percent High Density Polyethylene 50-60 percent Polyvinyl Chloride (PVC) 5-10 percent Low Density Polyethylene 5-10 percent Polypropylene Polystyrene All other resins 5-10 percent 5-10 percent 5-10 percent What Do the Numbers Mean? 1 -- PETE (Polyethylene terephthalate) •PET (or PETE) is used in the production of soft drink bottles, peanut butter jars... •PET can be recycled into fiberfill for sleeping bags, carpet fibers, rope, pillows... What Do the Numbers Mean? 2 -- HDPE (High-density polyethylene) •HDPE is found in milk jugs, butter tubs, detergent bottles, motor oil bottles... •HDPE can be recycled into flower pots, trash cans, traffic barrier cones, detergent bottles... What Do the Numbers Mean? 3 -- V (Polyvinyl chloride) •PVC is used in shampoo bottles, cooking oil bottles, fast food service items... •PVC can be recycled into drainage and irrigation pipes... What Do the Numbers Mean? 4 -- LDPE (Low-density polyethylene) •LDPE is found in grocery bags, bread bags, shrink wrap, margarine tub tops... •LDPE can be recycled into new grocery bags... What Do the Numbers Mean? 5 -- PP (Polypropylene) •PP is used in most yogurt containers, straws, pancake syrup bottles, bottle caps.... •PP can be recycled into plastic lumber, car battery cases, manhole steps... What Do the Numbers Mean? 6 -- PS (Polystyrene) •PS is found in disposable hot cups, packaging materials (peanuts), and meat trays... •PS can be recycled into plastic lumber, cassette tape boxes, flower pots... What Do the Numbers Mean? 7 -- Other •This is usually a mixture of various plastics, like squeeze ketchup bottles, "microwaveable" dishes... Timeline of Plastics 1862 – First man-made plastic 1866 – Celluloid makes it’s debut 1891 – Rayon is discovered 1907 – Bakelite is invented 1913 – Cellophane causes the plastics craze Timeline of Plastics 1926 – PVC is invented 1933 – Polyethylene is discovered 1933 – Saran makes it’s debut 1938 – Teflon is discovered 1939 – Nylon stockings hit market 1957 – Here comes velcro