Actual yield
advertisement

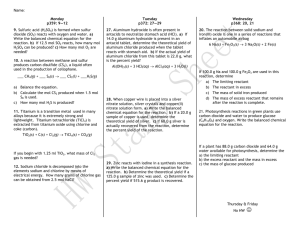
April 7, 2014 • Today: Stoichiometry and % Yield Percent Yield • Remember, stoichiometry is used to tell you how much product you can form from X amount of product without doing the reaction • Percent yield tells you how much product you actually got in the lab compared to how much you could have got Theoretical yield • The maximum amount of product that can be formed from a given amount of reactant. – This is a value you calculate on paper • In other words: (Write your own definition of Theoretical yield here) Actual yield • The measured amount of a product obtained from a reaction – This is a measurement of a product formed in an actual chemical reaction • In other words: (Write your own definition of Actual yield here) Percent Yield Formula Actual yield x 100 = Theoretical yield Example 1 Mg(s) + 2H2O(g) Mg(OH)2(s) + H2(g) A. If 16.2 g Mg are heated with excess H2O how many grams of hydrogen gas could theoretically be formed? Known: mass Mg Unknown: mass H2 Plan: g Mg mol Mg mol H2 g H2 Relationships: molar mass Mg = 24.31 g/mol, molar mass H2 = 2.02 g/mol, mole ratio = 1 mol Mg : 1 mol H2 16.2 g Mg | 1 mol Mg | 1 mol H2 | 2.02 g H2 = 16.2 x 2.02 = 1.35 g 24.31 g 1 mol Mg 1 mol H2 24.31 Example 1 B. If only 0.905 g H2 are actually formed, what is the percent yield of this reaction? Actual yield x 100 = % yield Theoretical yield 0.905 g H2 1.35 g H2 x 100 = 67.0% (This means that you produced or collected only 67% of the product that it was possible to form with the amount of reactant you started with.) Example 2 CO(g) + 2H2(g) CH3OH(l) If 11.0 g H2 reacts with CO to produce 68.4 g CH3OH, what is the percentage yield of CH3OH? We need to determine the theoretical yield. Known: mass H2 Unknown: mass CH3OH Plan: g H2 mol Mg mol H2 g H2 Relationships: molar mass H2 = 2.02 g/mol molar mass CH3OH = 32.04 g/mol mole ratio = 2 mol H2 : 1 mol CH3OH Example 2 CO(g) + 2H2(g) CH3OH(l) If 11.0 g H2 reacts with CO to produce 68.4 g CH3OH, what is the percentage yield of CH3OH? Known: mass H2 Unknown: mass CH3OH Plan: g H2 mol Mg mol H2 g H2 Relationships: molar mass H2 = 2.02 g/mol, molar mass CH3OH = 32.04 g/mol, mole ratio = 2 mol H2 : 1 mol CH3OH 11.0 g H2 | 1 mol H2 | 1 mol CH3OH | 32.04 g CH3OH = 11.0 x 32.04 = 87.2 2.02 H2 2 mol H2 1 mol CH3OH 2.02 x 2 g Example 2 CO(g) + 2H2(g) CH3OH(l) If 11.0 g H2 reacts with CO to produce 68.4 g CH3OH, what is the percentage yield of CH3OH? Actual yield x 100 = % yield Theoretical yield 68.4 g CH3OH 87.2 g CH3OH x 100 = 78.4%