enzymes which hydrolyse the b
advertisement
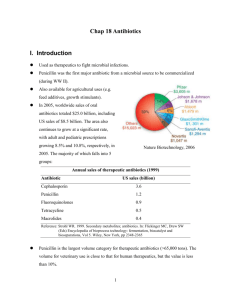
Antibiotics Step 1: How to Kill a Bacterium. • What are the bacterial weak points? • Specifically, which commercial antibiotics target each of these points? Target 1: The Bacterial Cell Envelope Two types of bacteria • Gram-positive: Stained dark blue by Gram-staining procedure • Gram-negative: Don’t take up the crystal violet stain, and take up counterstain (safranin) instead, staining pink in the Gram procedure. Structure of the bacterial cell envelope. Gram-positive. Gram-negative. Gram staining animation http://student.ccbcmd.edu/courses/bio141/labmanua/lab6/imag es/gram_stain_11.swf Structure of peptidoglycan. Peptidoglycan synthesis requires cross-linking of disaccharide polymers by penicillin-binding proteins (PBPs). NAMA, N-acetylmuramic acid; NAGA, N-acetyl-glucosamine. Antibiotics that Target the Bacterial Cell Envelope Include: • The b-Lactam Antibiotics • Vancomycin • Daptomycin Target 2: The Bacterial Process of Protein Production An overview of the process by which proteins are produced within bacteria. Structure of the bacterial ribosome. Antibiotics that Block Bacterial Protein Production Include: • • • • • • • • Rifamycins Aminoglycosides Macrolides and Ketolides Tetracyclines and Glycylcyclines Chloramphenicol Clindamycin Streptogramins Linezolid (member of Oxazolidinone Class) Target 3: DNA and Bacterial Replication Bacterial synthesis of tetrahydrofolate. Supercoiling of the double helical structure of DNA. Twisting of DNA results in formation of supercoils. During transcription, the movement of RNA polymerase along the chromosome results in the accumulation of positive supercoils ahead of the enzyme and negative supercoils behind it. (Adapted with permission from Alberts B, Johnson A, Lewis J, et al. Molecular Biology of the Cell. New York: Garland Science, 2002:314.) Replication of the bacterial chromosome. A consequence of the circular nature of the bacterial chromosome is that replicated chromosomes are interlinked, requiring topoisomerase for appropriate segregation. Antibiotics that Target DNA and Replication Include: • Sulfa Drugs • Quinolones • Metronidazole Which Bacteria are Clinically Important? General Classes of Clinically Important Bacteria Include: • • • • • • Gram-positive aerobic bacteria Gram-negative aerobic bacteria Anaerobic bacteria (both Gram + and -) Atypical bacteria Spirochetes Mycobacteria Gram-positive Bacteria of Clinical Importance • Staphylococci – Staphylococcus aureus – Staphylococcus epidermidis Staphylococcus aureus • Streptococci – – – – Streptococcus pneumoniae Streptococcus pyogenes Streptococcus agalactiae Streptococcus viridans • Enterococci – Enterococcus faecalis – Enterococcus faecium • Listeria monocytogenes • Bacillus anthracis Streptococcus viridans Gram-negative Bacteria of Clinical Importance • Enterobacteriaceae – Escherichia coli, Enterobacter, Klebsiella, Proteus, Salmonella, Shigella, Yersinia, etc. • Pseudomonas aeruginosa • Neisseria – Neisseria meningitidis and Neisseria gonorrhoeae • Curved Gram-negative Bacilli – Campylobacter jejuni, Helicobacter pylori, and Vibrio cholerae • • • • Haemophilus Influenzae Bordetella Pertussis Moraxella Catarrhalis Acinetobacter baumannii Anaerobic Bacteria of Clinical Importance • Gram-positive anaerobic bacilli – Clostridium difficile – Clostridium tetani – Clostridium botulinum • Gram-negative anaerobic bacilli – Bacteroides fragilis Atypical Bacteria of Clinical Importance Include: • • • • • • Chlamydia Mycoplasma Legionella Brucella Francisella tularensis Rickettsia Spirochetes of Clinical Importance Include: • Treponema pallidum • Borrelia burgdorferi • Leptospira interrogans Mycobacteria of Clinical Importance Include: • Mycobacterium tuberculosis • Mycobacterium avium • Mycobacterium leprae Antibiotics that Target the Bacterial Cell Envelope • The b-Lactam Antibiotics Mechanism of action of β-lactam antibiotics. Normally, a new subunit of Nacetylmuramic acid (NAMA) and N-acetylglucosamine (NAGA) disaccharide with an attached peptide side chain is linked to an existing peptidoglycan polymer. This may occur by covalent attachment of a glycine () bridge from one peptide side chain to another through the enzymatic action of a penicillin-binding protein (PBP). In the presence of a β-lactam antibiotic, this process is disrupted. The β-lactam antibiotic binds the PBP and prevents it from cross-linking the glycine bridge to the peptide side chain, thus blocking incorporation of the disaccharide subunit into the existing peptidoglycan polymer. Mechanism of penicillin-binding protein (PBP) inhibition by β-lactam antibiotics. PBPs recognize and catalyze the peptide bond between two alanine subunits of the peptidoglycan peptide side chain. The β-lactam ring mimics this peptide bond. Thus, the PBPs attempt to catalyze the β-lactam ring, resulting in inactivation of the PBPs. Six P's by which the action of βlactams may be blocked: (1) penetration, (2) porins, (3) pumps, (4) penicillinases (βlactamases), (5) penicillin-binding proteins (PBPs), and (6) peptidoglycan. The Penicillins Category Parenteral Agents Oral Agents Natural Penicillins Penicillin G Penicillin V Antistaphylococcal penicillins Nafcillin, oxacillin Dicloxacillin Aminopenicillins Ampicillin Amoxicillin and Ampicillin Aminopenicillin + blactamase inhibitor Ampicillin-sulbactam Amoxicillin-clavulanate Extended-spectrum penicillin Piperacillin, ticaricillin Carbenicillin Extended-spectrum penicillin + b-lactamase inhibitor Piperacillin-tazobactam, ticaricillin-clavulanate INTRODUCTION • • • • • • • • • • Antibacterial agents which inhibit bacterial cell wall synthesis Discovered by Fleming from a fungal colony (1928) Shown to be non toxic and antibacterial Isolated and purified by Florey and Chain (1938) First successful clinical trial (1941) Produced by large scale fermentation (1944) Structure established by X-Ray crystallography (1945) Full synthesis developed by Sheehan (1957) Isolation of 6-APA by Beecham (1958-60) - development of semi-synthetic penicillins Discovery of clavulanic acid and b-lactamase inhibitors http://www.microbelibrary.org/microbelibrary/files/ccImages/Articl eimages/Spencer/spencer_cellwall.html STRUCTURE R= O CH2 C Benzyl penicillin (Pen G) H S Me 6-Aminopenicillanic acid (6-APA) R R= O H H N Acyl side chain CH2 N Me O CO2H b-Lactam ring Phenoxymethyl penicillin (Pen V) Thiazolidine ring Side chain varies depending on carboxylic acid present in fermentation medium CH2 CO2H Penicillin G present in corn steep liquor OCH2 CO2H Penicillin V (first orally active penicillin) Shape of Penicillin G O C R Me H NH S Me O H N H CO2H .. Folded ‘envelope’ shape Properties of Penicillin G • • • • • • • Active vs. Gram +ve bacilli and some Gram -ve cocci Non toxic Limited range of activity Not orally active - must be injected Sensitive to b-lactamases (enzymes which hydrolyse the b-lactam ring) Some patients are allergic Inactive vs. Staphylococci Drug Development Aims • To increase chemical stability for oral administration • To increase resistance to b-lactamases • To increase the range of activity SAR Amide essential O C H N Cis Stereochemistry essential H H S R Me N O b Lactam essential CO2H Conclusions • • • • • • • Me Carboxylic acid essential Bicyclic system ess ential Amide and carboxylic acid are involved in binding Carboxylic acid binds as the carboxylate ion Mechanism of action involves the b-lactam ring Activity related to b-lactam ring strain (subject to stability factors) Bicyclic system increases b-lactam ring strain Not much variation in structure is possible Variations are limited to the side chain (R) Mechanism of action • Penicillins inhibit a bacterial enzyme called the transpeptidase enzyme which is involved in the synthesis of the bacterial cell wall The b-lactam ring is involved in the mechanism of inhibition Penicillin becomes covalently linked to the enzyme’s active site leading to irreversible inhibition • • O C H H N S R N Nu O H Me Me O Enz C CO2H H H N R Enz-Nu -H O H S N Me Me O H CO2H C H H N H R O C HN Nu-Enz S Me Me CO2H Covalent bond formed to transpeptidase enzyme Irreversible inhibition Mechanism of action - bacterial cell wall synthesis NAM L-Ala D-Glu L-Lys NAG NAM L-Ala D-Glu L-Lys Bond formation inhibited by penicillin NAM L-Ala NAG D-Glu NAM L-Lys L-Ala NAG NAM L-Ala NAG D-Glu L-Lys NAM L-Ala NAG NAM D-Glu L-Ala NAM NAG L-Lys NAM D-Glu L-Ala L-Lys L-Ala D-Glu D-Glu D-Glu L-Lys L-Lys L-Lys Mechanism of action - bacterial cell wall synthesis NAM NAG NAM L-Ala L-Ala D-Glu D-Glu L-Lys Gly Gly Gly Gly Gly NAG L-Lys D-Ala D-Ala D-Ala D-Ala SUGAR BACKBONE Gly Gly Gly Gly Gly PENICILLIN D-Alanine NAM NAG TRANSPEPTIDASE NAM L-Ala L-Ala D-Glu D-Glu L-Lys D-Ala Gly Gly Gly Gly Gly Cross linking L-Lys D-Ala NAG Gly Gly Gly Gly Gly SUGAR BACKBONE Mechanism of action - bacterial cell wall synthesis • Penicillin inhibits final crosslinking stage of cell wall synthesis • It reacts with the transpeptidase enzyme to form an irreversible covalent bond • Inhibition of transpeptidase leads to a weakened cell wall • Cells swell due to water entering the cell, then burst (lysis) • Penicillin possibly acts as an analogue of the L-Ala-g-D-Glu portion of the pentapeptide chain. However, the carboxylate group that is essential to penicillin activity is not present in this portion Mechanism of action - bacterial cell wall synthesis Alternative theory- Pencillin mimics D-Ala-D-Ala. Normal Mechanism Pe ptide Chain D-Ala OH Pe ptide Chain Pe ptide Chain D-Ala CO 2H Pe ptide Chain D-Ala Gly D-Ala O H OH Pe ptide Chain Gly Mechanism of action - bacterial cell wall synthesis Alternative theory- Penicillin mimics D-Ala-D-Ala. Mechanism inhibited by penicillin Blocked Pe ptide Chain Blocked H2O O R C O H NH S N O H OH Me Me R O Gly C NH H O R S NH H O HN HN Me CO2H O C Me CO2H Blocked O S Me Me CO2H Irreversibly blocked Mechanism of action - bacterial cell wall synthesis Penicillin can be seen to mimic acyl-D-Ala-D-Ala R R C H N H H S C Me O H N H H N O N O Me O CO2H Penicillin Me H CH3 CO2H Acyl-D-Ala-D-Ala Penicillin Analogues - Preparation 1) By fermentation • vary the carboxylic acid in the fermentation medium • limited to unbranched acids at the a-position i.e. RCH2CO2H • tedious and slow 2) By total synthesis • only 1% overall yield (impractical) 3) By semi-synthetic procedures • Use a naturally occurring structure as the starting material for analogue synthesis Penicillin Analogues - Preparation O H N C H S CH2 Me Penicillin G N Me O CO2H Penicillin acylase or chemical hydrolysis H2N Fermentation H H S N Me Me O 6-APA CO2H O R C Cl O C H H N H S Me R N Semi-synthetic penicillins Me O CO2H Penicillin Analogues - Preparation Problem - How does one hydrolyse the side chain by chemical means in presence of a labile b-lactam ring? Answer - Activate the side chain first to make it more reactive PhCH2 O C NH S ROH PhCH2 C N PEN N O PCl5 OR Cl H2O PhCH2 C N PEN 6-APA CO2H Note - Reaction with PCl5 requires involvement of nitrogen’s lone pair of electrons. Not possible for the b-lactam nitrogen. Problems with Penicillin G • It is sensitive to stomach acids • It is sensitive to b-lactamases enzymes which hydrolyse the blactam ring • it has a limited range of activity Problem 1 - Acid Sensitivity Reasons for sensitivity 1) Ring Strain O C H N H H S R Me Acid or enzyme O O C H N N Me O CO2H H S R HO H2O H N Me Me C H N H H S R HO2C HN Me Me O H CO2H CO2H Relieves ring strain Problem 1 - Acid Sensitivity Reasons for sensitivity 2) Reactive b-lactam carbonyl group Does not behave like a tertiary amide Tertiary amide R R C R C NR2 O O b-Lactam Unreactive N R Me S S Me Me O N CO2H H Folded ring system • • X N Me O CO2H Impossibly strained Interaction of nitrogen’s lone pair with the carbonyl group is not possible Results in a reactive carbonyl group Problem 1 - Acid Sensitivity Reasons for sensitivity 3) Acyl Side Chain - neighbouring group participation in the hydrolysis mechanism R H C N H S O N O N R S N R S -H O N O O HN O H Further reactions Problem 1 - Acid Sensitivity Conclusions • • • The b-lactam ring is essential for activity and must be retained Therefore, cannot tackle factors 1 and 2 Can only tackle factor 3 Strategy Vary the acyl side group (R) to make it electron withdrawing to decrease the nucleophilicity of the carbonyl oxygen H N E.W.G. H S C N O Decreases nucleophilicity O Problem 1 - Acid Sensitivity Examples PhO X H N CH2 H S C electronegative oxygen • • • • HC N Better acid stability and orally active But sensitive to b-lactamases Slightly less active than Penicillin G Allergy problems with some patients H S N O O Penicillin V (orally active) H N C R O a O X = NH 2, Cl, PhOCONH, Heterocycles, CO2H • Very successful semisynthetic penicillins e.g. ampicillin, oxacillin Natural penicillins include Penicillin G (parenteral) and Penicillin V (oral) Gram-positive bacteria Streptococcus pyogenes, Viridans group streptococci, Some Streptococcus pneumoniae, Some Enterococci, Listeria monocytogenes Gram-negative bacterai Neisseria meningitidis, Some Haemophilus influenzae Anaerobic bacteria Clostridia spp. (except C. difficile), Antinomyces israelii Spirochetes Treponema pallidum Leptospira spp. Problem 2 - Sensitivity to b-Lactamases Notes on b-Lactamases • Enzymes that inactivate penicillins by opening b-lactam rings • Allow bacteria to be resistant to penicillin • Transferable between bacterial strains (i.e. bacteria can acquire resistance) • Important w.r.t. Staphylococcus aureus infections in hospitals • 80% Staph. infections in hospitals were resistant to penicillin and other antibacterial agents by 1960 • Mechanism of action for lactamases is identical to the mechanism of inhibition for the target enzyme • But product is removed efficiently from the lactamase active site O O C H N H C S R N O CO2H H S Me Me H N R b-Lactamase HO2C HN Me Me CO2H Problem 2 - Sensitivity to b-Lactamases Strategy • Block access of penicillin to active site of enzyme by introducing bulky groups to the side chain to act as steric shields • Size of shield is crucial to inhibit reaction of penicillins with blactamases but not with the target enzyme (transpeptidase) O Bulky group C H N H H S Me R N Enzyme Me O CO2H Problem 2 - Sensitivity to b-Lactamases Examples - Methicillin (Beecham - 1960) ortho groups important O MeO C H N H H S N OMe Me Me O CO2H • • • • • • • Methoxy groups block access to b-lactamases but not to transpeptidases Active against some penicillin G resistant strains (e.g. Staphylococcus) Acid sensitive (no e-withdrawing group) and must be injected Lower activity w.r.t. Pen G vs. Pen G sensitive bacteria (reduced access to transpeptidase) Poorer range of activity Poor activity vs. some streptococci Inactive vs. Gram - bacteria Problem 2 - Sensitivity to b-Lactamases Examples - Oxacillin R' O C R N O H H S N Me Bulky and e- withdrawing • • • • • • • • H N Me Oxacillin R = R' = H Cloxacillin R = Cl, R' = H Flucloxacillin R = Cl, R' = F Me O CO2H Orally active and acid resistant Resistant to b-lactamases Active vs. Staphylococcus aureus Less active than other penicillins Inactive vs. Gram - bacteria Nature of R & R’ influences absorption and plasma protein binding Cloxacillin better absorbed than oxacillin Flucloxacillin less bound to plasma protein, leading to higher levels of free drug Antistaphylococcal Penicillins include Nafcillin and Oxacillin (parenteral) as well as Dicloxacillin (oral) Gram-positive bacteria Some Staphylococcus aureus, Some Staphylococcus epidermidis Problem 3 - Range of Activity Factors 1. Cell wall may have a coat preventing access to the cell 2. Excess transpeptidase enzyme may be present 3. Resistant transpeptidase enzyme (modified structure) 4. Presence of b-lactamases 5. Transfer of b-lactamases between strains 6. Efflux mechanisms Strategy • The number of factors involved make a single strategy impossible • Use trial and error by varying R groups on the side chain • Successful in producing broad spectrum antibiotics • Results demonstrate general rules for broad spectrum activity. Problem 3 - Range of Activity Results of varying R in Pen G 1. R= hydrophobic results in high activity vs. Gram + bacteria and poor activity vs. Gram - bacteria 2. Increasing hydrophobicity has little effect on Gram + activity but lowers Gram - activity 3. Increasing hydrophilic character has little effect on Gram + activity but increases Gram - activity 4. Hydrophilic groups at the a-position (e.g. NH2, OH, CO2H) increase activity vs Gram - bacteria Problem 3 - Range of Activity Examples of Aminopenicillins include: Class 1 - NH2 at the a-position Ampicillin and Amoxicillin (Beecham, 1964) H H NH2 HO C C H N NH2 C H C H N H O O O Ampicillin (Penbritin) 2nd most used penicillin O Amoxicillin (Amoxil) Problem 3 - Range of Activity Examples of Aminopenicillins Include: Properties • Active vs Gram + bacteria and Gram - bacteria which do not produce b-lactamases • Acid resistant and orally active • Non toxic • Sensitive to b-lactamases • Increased polarity due to extra amino group • Poor absorption through the gut wall • Disruption of gut flora leading to diarrhea • Inactive vs. Pseudomonas aeruginosa •Amoxicillin is sometimes used together with clarithromycin (Biaxin) to treat stomach ulcers caused by Helicobacter pylori, a Gram - bacteria •Also, a stomach acid reducer (lansoprazole, or Prevacid) is sometimes added. Helicobacter pylori Helicobacter pylori is linked to stomach inflammation, which may also result in gastric ulcers and stomach cancer •In the early 20th century, ulcers were believed caused by stress •In 1982, Robin Warren and Barry Marshall, two Australian physicians, suggested link between H. pylori and ulcers •Medical community was slow to accept (first abstract describing such results was rejected for a poster) In 2005, the two researchers, Barry Marshall and J. Robin Warren, received the Nobel Prize in medicine for their discovery of the bacterium Helicobacter pylori and its role in gastritis and peptic ulcer disease Problem 3 - Range of Activity Prodrugs of Ampicillin (Leo Pharmaceuticals - 1969) O H R= NH2 C CH2O CMe3 PIVAMPICILLIN C C H N O H H S O Me R= TALAMPICILLIN O Me N O O CO2R R= CH Me O C O CH2Me BACAMPICILLIN Properties • Increased cell membrane permeability • Polar carboxylic acid group is masked by the ester • Ester is metabolised in the body by esterases to give the free drug Problem 3 - Range of Activity Mechanism H PEN O H PEN H C C O CH2 O C O CH2 CMe3 ENZYME C OH O O O O • • • PEN Formaldehyde Ester is less shielded by penicillin nucleus Hydrolysed product is chemically unstable and degrades Methyl ester of ampicillin is not hydrolysed in the body - bulky penicillin nucleus acts as a steric shield The aminopenicillins include Ampicillin (parenteral) as well as Amoxicillin and Ampicillin (both oral) Gram-positive bacteria Streptococcus pyogenes, Viridans streptococci, Some Streptococcus pneumoniae, Some enterococci Listeria monocytogenes Gram-negative bacteria Neisseria meningitidis, Some Haemophilus influenzae, Some Enterobacteriaceae Anaerobic bacteria Clostridia spp. (except C. difficile), Antinomyces israelii Spirochetes Borrelia burgdorferi