Acute Renal Failure - Professional Portfolio
advertisement

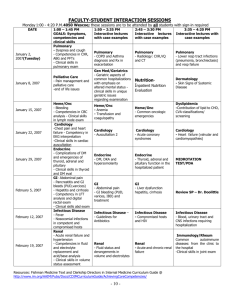
Running Head: CASE STUDY NUR 7201 1&2 Case Study NUR 7201 1&2 Ashley Peczkowski Wright State University NUR 7201 1 CASE STUDY NUR 7201 1&2 2 Case Study NUR 7201 Case Study One Questions: 1. What is the most likely diagnoses for this patient and what are the criteria for this diagnosis? What is your rationale? The patient’s most likely diagnosis is pulmonary atrial hypertension (PAH) secondary to anorexigen pill consumption. Idiopathic PAH is more common in women and remains the most common type. Primary PAH includes idiopathic, familial, and drug or toxin induced. Other causes of PAH include congenital heart disease, connective tissue diseases, toxins, drugs, human immunodeficiency (HIV), portal hypertension, hemoglobinopathies, and myeloproliferative disorders. These causes are considered Group I PAH; Group II includes PAH with left heart failure; Group III includes PAH with lung disease or hypoxemia; Group IV, PAH from chronic thrombotic or embolic diseases; and Group V is miscellaneous PAH. According to the European Society of Cardiology guidelines (Albert-Barbera et al., 2009), the criteria for a pulmonary atrial hypertension (PAH) diagnosis is an increase in mean pulmonary arterial pressure of ≥25mmHG at rest with a normal capillary wedge pressure, left atrial pressure, left ventricular end-diastolic pressure, and a pulmonary vascular resistance pressure greater than three Wood units. A right heart catheterization (RHC) is the gold standard for diagnosis. This test has the highest sensitivity (93%) and specificity (95%) and is important for measuring severity of hemodynamic impairment, predict prognosis, identify other causes, monitor etiopathology, and evaluate right ventricular function (Schannwell, Steiner, & Strauer, 2007). During the RHC a vasoreactivity test can be performed to identify patients who might benefit from long term therapy with calcium channel blockers. An acute responder has a reduced mean pulmonary arterial pressure by CASE STUDY NUR 7201 1&2 3 10mm/Hg to 40mm/Hg without reduced cardiac output (McLaughlin et al., 2009). The current drug of choice for vasoreactivity testing is nitric oxide. Because this test is invasive, prediagnosis tests are performed to confirm PAH and rule out other differential diagnoses (AlbertBarbera et al., 2009). The rational for this patient’s diagnosis came from the physical assessment and the prediagnosis test results. The patient presented with increasing shortness of breath and fatigue, presyncope episodes, dyspneic when walking short distances with relief after sitting, and substernal chest pain. Advanced disease is suggested through a left parasternal lift, prominent S2, RV S4, clear lung fields, and an increased jugular a wave indicating elevated right ventricular pressure, hypertrophy and decreased right ventricular compliance; grade III/VI holosystolic murmur that is increased with respiration indicates tricuspid regurgitation; hepatojugular reflux shows signs of high central venous pressures. The cyanotic lips (central cyanosis) and ankle edema suggest hypoxia and reduced cardiac output. The physical data are suggestive of a cardiopulmonary disease such as right ventricular heart failure/hypertrophy, pulmonary embolism, severe anemia or many others. History included use of known toxins such as the fenfluramine and phentermine which have been indicated in causing primary PAH. Because of this data PAH is suspected; an electrocardiogram (EKG) and chest x-ray re-confirm this diagnosis. There are many EKG changes seen in patients with right ventricular hypertrophy (RVH) such as prolonged QRS duration, right axis deviation, and enlarge right atrium. The diagnosis is suggested through prominent RSR in lead V1-V6, ST depression, and T wave inversion. Right precordial leads with ST depression and T inversion indicate that the RVH is severe. An echocardiogram (sensitivity 83%; specificity 72%) will provide the degree of severity. The enlarged pulmonary trunk seen on CASE STUDY NUR 7201 1&2 4 the chest x-ray and the absence of left ventricular enlargement on both the chest x-ray and EKG compliment this conclusion (MacLean, 2007). Fenfluramine and phentermine use have been associated with increased risk of PAH and have since been removed from the market. The exact correlation between these types of anorexigen pills and PAH are not fully understood, however research suggests that these drugs affects the serotonin receptors in the pulmonary endothelium. Research hypothesis that: While normal serotonin synthesized by the pulmonary endothelium has a rate-limiting step, the use of anorexigen pills causes an override initiating unregulated proliferation and/ or constriction leading to PAH. During this process blood platelets are serotonin depleted while plasma serotonin is elevated; vasoconstrictors such as endothelin and thromboxane are increased while vasodilators like prostacyclin are decreased. Proliferation is also increased through endothelial injury exposing muscle to growth factors and mitogen, and releases proinflammatory cytokines. This process leads to elevated fibrinopeptide A, plasminogen activator inhibitor-1, and decreased tissue plasminogen creating a hypercoagulable state (McLaughlin et al., 2009; MacLean, 2007). This proliferation, apoptosis, and, in some patients, vasoconstriction results in constricted small pulmonary arterial abnormalities including intimal hyperplasia, medial hypertrophy, adventitial proliferation, thrombosis in situ, inflammation, and plexiform arteriopathy. Constriction in the pulmonary arterioles increases the peripheral vasculature resistance increasing the afterload. Marked increases in after load lead to right ventricular hypertrophy and dilatation. Key features in Group I are the elevated pulmonary arterial pressure (greater than 25mm/Hg) and a normal pulmonary capillary wedge pressure (McLaughlin et al., 2009). Based on the patients current presentation, results of EKG and chest x-ray, and history of fenfluramine and phentermine use, CASE STUDY NUR 7201 1&2 5 diagnosis of PAH is most likely. Additional pre-diagnosis testing and conformational diagnosis though right heart catheterization is recommended. 2. What diagnostic test should be performed? Explain your rationale? Pulmonary arterial hypertension is a diagnosis of exclusion and therefore the patient should obtain investigational testing to determine the clinical grouping and etiology of the diagnosis. Because the clinical presentation of PAH is non-specific and common in other cardiopulmonary disease states, several pre-diagnosis tests are needed. These tests include an EKG, chest x-ray, pulmonary function tests (PFT), arterial blood gases (ABG), transthoracic echocardiography (TTE), ventilation/ perfusion lung scan (VQ scan), contrast computed tomography (CT) scan, possible pulmonary angiography, cardiac magnetic resonance imaging (MRI), blood tests including complete blood count (CBC), basic metabolic panel (BMP), thyroid functioning tests, serological testing for HIV, hepatitis, and connective tissue disease (CTD); abdominal ultrasound, and finally right heart catheterization and vasoreactivity. The European Society of Cardiology and the European Respiratory Society have developed a PAH algorithm guideline to help practitioners accurately diagnosis PAH. If the physical assessment and presenting symptoms are consistent with PAH then an EKG, chest x-ray, TTE, PFT, ABG, and chest CT should be ordered. Once other cardiopulmonary diseases and hypoxia are ruled out then a VQ scan is ordered. Finally once any other causes have been ruled out a right heart catheterization should be ordered to confirm PAH diagnosis. If diagnosis is confirmed than a vasoreactivity test can be completed during catheterization to determine if calcium channel blockers are an effective treatment measure. Further testing to determine clinical grouping and cause should be considered. The specific diagnostic tests included the CBC, BMP, Liver function tests, abdominal US, HIV, hepatitis, CTD, and history of drug toxins (Albert-Barbera et al., CASE STUDY NUR 7201 1&2 6 2009). Once the diagnosis is confirmed and the clinical grouping identified then the correct treatment can be initiated. 3. What is the appropriate therapy for this patient. Include all types of therapy and rationale for your choices. A large majority of therapy for this patient is reducing morbidity and prolonging mortality. There is a high mortality rate of 15% within the first year on medical therapy. Poor prognosis is predicted through: advanced functional class, reduced 6-minute walking test, high right atrial pressures, significant right ventricular dysfunction and failure, reduced cardiac index, elevated brain natriuretic peptide, and an underlying scleroderma disease. The main treatment goal is to reduce symptoms, improve quality of life, and improve survival. General measure prevention therapy include physical rehabilitation, pregnancy prevention, social support for depression and anxiety, flu and pneumonia vaccine for infection prevention, and epidural use for elective surgeries. Supportive therapy includes oral anticoagulation due to the high risk of vascular thrombotic lesions, diuretics for fluid retention, oxygen for hypoxemia, and digoxin for improved cardiac output in acute atrial tachyarrhythmias. Once the vasoreactivity test has been performed and the test is positive then the patient can be placed on specific calcium channel blocker therapy. Calcium channel blockers used in treatment for PAH are nifedipine, diltiazem, or amlodipine (McLaughlin et al., 2009). For this patient because of her marked dyspnea with minimal activity, fatigue with resting, right sided heart failure, and her near syncope episode, she is placed in a New York Heart Association (NYHA) class IV classification. Based on the World Health Organization functional class (WHO-FC) this patient should be placed on one or a combinations of CASE STUDY NUR 7201 1&2 7 Ambrisentan, Bosentan, Sildenafil, Epoprostenol IV, or Iloprost inhaled. These drugs are a 1-A recommendation. Combination therapy is recommended for PAH patients of advanced classification. Warfarin is recommended to reduce hypercoagulable state and prevent emboli; a therapeutic INR is between 1.5-2.5; symptomatic management from right ventricular fluid overload involves the use of Diuretics; oxygen therapy is initiated to maintain O2 stats greater than 90%; and lastly calcium channel blockers improves right ventricular compliance for those with positive vasodilator response. Epoprostenol IV continuous infusion through a port is indicated for those patients with class IV symptoms (Albert-Barbert et al., 2009). According to the Ohio Board of Nursing (OBN) an acute care nurse practitioner (NP) may prescribe oxygen, calcium channel blockers, and diuretics. Warfarin may be prescribed per institutional protocol and physician initiated or consulted. Epoprostenol may only be prescribed if it has been physician initiated or consulted and the NP works within the specialty clinic setting (OBN, 2013). Epoprostenol is given 20 to 40 ng/kg/min for optimal therapy benefits. This drug is a prostacyclin which causes vasodilation and platelet aggregation. There may also be a cytoprotective and antiproliferative activity. This is the only drug in the treatment on PAH to show any improvement of mortality rates. Additional pharmacological therapy can be added if there is no improvement, minimal improvement, or worsening of condition (Albert-Barbert et al., 2009). Iloprost is an inhaled prostacyclin useful for improvement of exercise capacity and can be prescribed by an NP (Albert-Barbera et al., 2009; OBN, 2013). Ambrisentan and Bosentan are both endothelin receptor antagonist that cause vasodilation and release of antiproliferative substances like nitric oxide and prostacyclin which are helpful in the treatment of PAH (AlbertBarbera et al., 2009). Endothelin receptor antagonist may only be physician initiated or consulted prior to being prescribed by a NP (OBN, 2013). Lastly Sildenafil is a Phosphodiesterate type-5 CASE STUDY NUR 7201 1&2 8 inhibitor that also causes vasodilation and can be prescribed by a NP (Albert-Barbera, et al. 2009; OBN, 2013). Surgical treatment for PAH are reserved for patients with declining functional capacity despite maximal medical therapy. Surgical treatment involves atrial septostomy creating a right to left shunt; this allows for decreased right ventricular filling, improved right sided function, improve left sided filling, and improved cardiac output despite reduced systemic arterial oxygenation. Atrial septostomy is performed to provide palliation, restoration, and maintenance until a heart and lung transplant can be performed. Other treatments include pulmonary thromboendarterectomy and right ventricular assist devices (McLaughlin et al., 2009). The objective of medical therapy is to improve functional capacity, stabilize or improve hemodynamics, and improve survival. The easiest test to perform to evaluate effectiveness of therapy is the “6 minute walk test”. Improvement in walking is generally indicative of effective therapy treatment. Other routine tests to monitor drug therapies and PAH disease course include an EKG, 6 minute walk test, and a BNP every three to six months. If therapy is changed or there is a clinical worsening of the patient then an EKG, six minute walk test, cardio-pulmonary exercise test, BNP, echocardiography, and right heart catheterization should be performed every three to four months (Albert-Barbera et al., 2009). CASE STUDY NUR 7201 1&2 9 Case Study Two Questions: 1. What is your differential diagnosis? Explain. Differential diagnosis is based on the type of acute renal failure including prerenal, postrenal and intrinsic renal failure. Differential diagnoses for intrinsic renal failure are acute tubular necrosis (ATN), glomerulonephritis, and tubulointerstitial nephritis. Based on the patients recent history of receiving radiographic contrast during coronary angiography within 24 to 72 hours of oliguria, acute tubular necrosis from radiographic contrast, also called contrast induce nephropathy (CIN), is the most likely cause. CIN is indicated when the serum creatinine (Cr) is 0.5mg/dl or greater or Cr increases by 25% in 48 hours after radiographic contrast is given (Adam et al., 2006). Acute tubular necrosis can be caused by nephrotoxic drugs such as aminoglycosides, radiographic contrast, and chemotherapy or ischemia from hypotension or vascular occlusions. Glomerulonephritis develops from post infectious, immune diseases, vasculitis, hemolytic syndromes, or atheroembolic syndrome and presents with proteinuria and microscopic hematuria (Parmar, 2009). Tubulointerstitial nephritis can be caused by medications such as cephalosporins, methicillin, and rifampin or infections such as pyelonephritis and HIV (Gonzalez & Praga, 2010). Based on the patients history and physical both glomerulonephritis and tubulointerstitial nephritis are a less likely cause than acute tubular necrosis. Prerenal can be caused by volume depletion, reduced arterial blood flow, medications such as aspirin, NSAIDs, or ACE inhibitors, and decreased cardiac output. This patient is at risk for prerenal failure because of his prior medication use of aspirin and ACE inhibitors. Post renal failure results from an obstruction somewhere in the urinary system from a crystal, stone, or tumor (Peacock & Sinert, 2011). CASE STUDY NUR 7201 1&2 10 2. What is your next step to diagnose the problem? Explain. Of what value is a urinalysis and urinary electrolytes? A detailed history and physical is the most beneficial step in diagnosing renal failure because it allows you to narrow down renal failure type. Obtaining a serum cysteine C level and serum creatinine level next can show the degree of renal function. A serum cysteine C level is a more specific test to kidney failure because it shows an accurate glomerular filtration rate and is less influenced by variables such as age, race, sex, and muscle mass. For prerenal failure the UA results show a high specific gravity with normal sediment. Finally a urinalysis (UA) and urinary electrolytes are beneficial in creating a diagnosis. Post renal failure UA results have isosthenuria, specific gravity of 1.010, and various findings depending on cause such as hematuria or leukocytes. Intrinsic renal failure however varies greatly depending on cause. Typically all intrinsic renal failures have isosthenuria and some degree of proteinuria. The differentiating results are in the casts. Acute tubular necrosis has “muddy brown” granular casts, glomerulonephritis has hematuria and red blood cell casts, and tubulointerstitial nephritis has leukocytes, white blood cells, and eosinophil (Peacock & Sinert, 2011). Urinary electrolytes are less helpful in diagnosis unless the patient has oliguria prerenal failure or oliguria ATN. Pre-renal failure has a FEna that is less than one percent and urinary sodium of less than 20mEq/L. In ATN the FEna is greater than two percent with urinary sodium greater than 20mEq/L. These results vary considerably when the cause of acute renal failure is different than those stated above (Adam et al., 2006). Other urinary electrolytes can provide indications of other disease processes. These electrolytes include sodium, potassium, chloride, and calcium. Sodium can give indication for HTN, glomerulonephritis, hepatic-renal syndrome; potassium levels indicate diabetic acidosis, ATN, adrenal insufficiency, and CASE STUDY NUR 7201 1&2 11 hyper/hypoaldosteronism; chloride levels indicate hypokalemia, tubular acidosis, and acid-base imbalances; and finally calcium levels can indicate obstructive renal failure and parathyroid disease (Peacock & Sinert, 2011). 3. What are the indications for dialysis in AKI (acute kidney injury)? Be specific. The indication for dialysis in acute kidney injury include a BUN > 70 mg/dl in a symptomatic patient without renal functional improvement, severe metabolic acidosis indicated by a <7.2 pH, extensive fluid overload causing pulmonary edema, uncontrolled hypertension greater than 180/110, hyperkalemia greater than 6.5 mEq/L, uremic symptoms, and pericarditis; all of which are unresponsive to medical therapy (Klarenbach, Manns, Pannu, Tonelli, & Wiebe, 2008). Other variable indications are: Less severe previously stated criteria in combination with gastritis with hemorrhage, nausea, vomiting, decreased appetite, coma, stupor, severe lethargy or malaise, asterixis, tremor, seizures, bleeding diathesis, and pericarditis. Drug or toxin ingestion of dialyzable agents such as lithium, ethylene, glycol, theophylline, barbiturates, and many others are indications for hemodialysis. Hemodialysis is the preferred choice of dialysis because it can be done in emergent situations, there is a greater control of hemodynamics, and blood products can be given if needed (Abrao, Balbi, & Medicina, 2012). Guidelines for dialysis treatment indications vary and are influenced by the patient’s presentation and the practitioner’s previous medical experiences. Based on this patient’s lab results and presentation, dialysis would not recommended. Close monitoring of the patients BNP, EKG, and vitals are necessary. 4. Write a set of admitting orders for the patient. Be specific. Admission: Admit: ICU/ CICU, attending Dr. Renal Consult nephrology CASE STUDY NUR 7201 1&2 12 Diagnosis: Acute renal failure Secondary diagnosis: Coronary artery disease; diabetes Condition: Serious Vital Signs: VS every hour with a temperature every four hours. Continuous cardiac monitor Record rhythm every shift and with cardiac rhythm changes. Call practitioner: BP: SBP <90mmHg or >180mmHg HR: <50 or >120 BPM Respirations: <10 or >30 Temperature ≥ 101.5 Obtain stat EKG if a change in cardiac rhythm or patient complains of chest pain. Call practitioner if EKG is ordered. Place patient on oxygen for O2 less than 92%. Titrate for O2 greater than 92%. NKA Allergies: Activity: As tolerated, ambulate in hallways TID Obtain vital signs every hour Nursing: CASE STUDY NUR 7201 1&2 13 Obtain rhythm strip every eight hours Monitor arterial line site every four hours for bleeding Call practitioner if site is bleeding Titrate oxygen level to maintain oxygen stats greater or equal to 92% Neuro checks every four hours and as needed. Call practitioner if there is a change in the patient’s mental condition POC blood glucose checks AC&HS and at 3am. Call if patients’ blood sugar is less than 50 or over 400 Obtain daily weights Strict I&O Place foley catheter IS every hour while awake; C&DB every 2 hours while awake Cardiac diet/ Renal diet with low potassium to maintain a heart healthy diet Diet: and to prevent an increase in the serum potassium levels which may lead to the patient requiring dialysis. Medications: IV: Normal saline 0.9% at 125cc/hr IV continuous Medications: Novolog insulin sliding scale SQ ACHS and three am: Blood Glucose (mg/dL) Dose <70 Hypoglycemia Protocol CASE STUDY NUR 7201 1&2 14 70-130 0 131-180 4 181-240 8 241-300 10 301-350 12 351-400 16 400> Call NP Aspirin 81mg PO daily Rosuvastatin 20mg PO daily Heparin 5,000 units SQ Q8H Docusate 100mg PO BID Losartan 50mg PO daily; hold if blood pressure less than 110 systolic Resume beta-blocker dose and schedule; Hold if BP less than 100 systolic or HR less than 50 BPM; Call practitioner if medication held. PRN: Morphine 2mg IV Q2H PRN minimal pain Morphine 4mg IV Q2H PRN moderate pain Morphine 6mg IV Q2H PRN severe pain Roxicodone 5-10mg PO Q3H PRN moderate pain Zofran 4mg IV/PO Q6H PRN nausea/ vomiting Melatonin 5mg PO QHS PRN sleep Lactulose 10-20g TID PRN constipation CASE STUDY NUR 7201 1&2 15 Dextrose 50% 25ml IV as needed of glucose less than 50; recheck POC in 15 minutes. Give OJ if glucose between 70-50 and recheck POC in 15 minutes. I&O: Monitor every hour Place foley for accurate I&O Call practitioner if urine output is less than 30cc/hr over four hours. Stat CBC, sedimentation rate, serum cysteine C level, BMP, phosphate level, Labs: magnesium level, A1C level, lipid profile, ABG, UA, and urinary electrolytes for baseline labs Once MRSA by PCR per ICU protocol Once 24 hour urine Obtain CBC daily to monitor hemoglobin and a BMP with magnesium and phosphorus every six hours to closely monitor potassium and creatinine level Obtain daily urine FEna, UA, and osmolality. Special tests: Stat chest xray to monitor for pulmonary edema Obtain renal ultrasound with flow studies to rule out post renal obstruction and hydronephrosis Consider daily chest x-ray if pulmonary edema or infiltrates present Consult to nephrology for monitoring and to determine if the patient’s condition Consult: or change in condition warrants the need for dialysis. Consult cardiac rehab. CASE STUDY NUR 7201 1&2 16 Consult diabetic educator. At Discharge: The patient should be referred to an optometrist when discharge for follow up on the diabetic retinopathy Rational for medication prescribing include: The patient should be removed off of all diabetic oral medication especially if he is on metformin as this can increase to amount of renal damage. Subcutaneous insulin Novolog should be given with each meal after the blood sugar has been checked to allow for closer monitors of the blood sugar. The patient many remain on their dose of beta blocker unless the patient’s blood pressure declines in which case the dose should be reduced by half. Beta blockers should never be stopped as this increases the decline of heart failure. The aspirin dose should be low dose as a higher dose has not shown to have enhanced effectiveness. Start normal saline 0.9% at 125cc/hr continuous because studies have shown that hydration can reduce the severity of acute tubular necrosis from radiographic contrast. Normal saline is better than half normal saline because the high sodium count allows for better volume expansion and inhibits the renin-angiotensin pathway (Guo, Li, Wong, & Zhang, 2011). 5. Complete the following chart with values present in prerenal and acute renal failure. Be certain to reference the chart. Table 1. Differential Criteria of Pre-renal and Acute Renal Failure. Laboratory Test FeNa Prerenal Acute Renal Failure <1% >2% CASE STUDY NUR 7201 1&2 17 BUN to creatinine ratio >20:1 <10:1 Urine specific gravity >1.020 1.010-1.020 >500 250-300 <20mmol/L >40mmol/L Normal or Muddy brown Granular Casts Hyaline Casts or Renal Tubular Epithelial Urine osmolality, mOsm per kg Urine sodium concentration, mEq per L (mmol per L) Urine sediment Cells Table 1. Adapted from: (1) Hancock, 2005; (2) Harrison, T. R., & Longo, D. L. (2013). Harrison's manual of medicine. New York: McGraw-Hill Medical CASE STUDY NUR 7201 1&2 18 Reference Abrao, J., Balbi, A., & Ponce, D. (2012). Early initiation of dialysis: Mortality and renal function recovery in acute kidney injury patients. Jornal Brasileiro de Nefrologia, 34(4), 337-342. doi:10.5935/0101.2800.20120022 Adam, A., Becker, C. R., Davidson, C., Lameire, N., McCullough, P. A., & Stacul, F. (2006). Pathophysiology of contrast-induced nephropathy. American Journal of Cardiology, 98, 14-20. doi:10.1016/j.amjcard.2006.01.020Parmar, M. S. (2009). Progressive acute kidney injury following myocardial infarction: Cholesterol embolization. British Medical Journal Case Reports, 733-745. doi:10.1136/bcr.06.2008.0103 Albert-Barbera, J., Beghetti, M., Corris, P., Galie, N., Gaine, S., Gibbs, S. . . . & Zellweger, M. (2009). Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). European Heart Journal, 30, 2493-2537. doi:10.1093/eurheartj/ehp297 Gonzalez, E., & Praga, M. (2010). Acute interstitial nephritis. Kidney International, 77, 956-961. doi:10.1038/ki.2010.89 Guo, J., Li, Z., Wong, P., & Zhang, A. (2011). Pathophysiology of contrast-induce nephropathy. International Journal of Cardiology, 158, 186-192. doi:10.1016/j.ijcard.2011.06.115 Hancock, J. (2005). Renal. (2nd ed.), The practitioners pocket pal (23). Miami: MedMaster Incorporated. CASE STUDY NUR 7201 1&2 19 Harjola, V., Lassus, J., Nieminen, M., Peuhkurinen, K., Pulkki, K., Siirila-Waris, K., & Sund, R. (2010). Markers of renal function and acute kidney injury in acute heart failure: Definitions and impact on outcomes of the cardiorenal syndrome. European Heart Journal, 31(22), 2791-2798. doi:10.1093/eurheartj/ehj293 Harrison, T. R., & Longo, D. L. (2013). Harrison's manual of medicine. New York: McGrawHill Medical. Klarenbach, S., Manns, B., Pannu, N., Tonelli, M., & Wiebe, N. (2008). Renal replacement therapy in patients with acute renal failure. The Journal of the American Medical Association, 299(7), 793-805. doi:10.1001/jama.299.7.793 MacLean, M. R. (2007). Pulmonary hypertension and serotonin hypothesis: Where are we now? Internalational Journal of Clinical Practice, 61(156), 27-31. doi: 10.1111/j.17421241.2007.01497.x McLaughlin, V., Archer, S., Badesch, D., Barst, R., Farber, H., Linder, J., ... Varga, J. (2009, April). ACCF/AHA 2009 Expert Consensus Document on Pulmonary Hypertension. Journal of the American College of Cardiology, 53(17), 1573-1619. http://dx.doi.org/10.1016/j.jacc.2009.01.004 Ohio Board of Nursing. (2013). The formulary developed by the committee on prescriptive governance. Retrieved from http://www.nursing.ohio.gov Schannwell, C., Steiner, S., & Strauer, B. (2007). Diagnostics in pulmonary hypertension. Journal of Physiology and Pharmacology, 58(5), 591-602. Retrieved from: http://www.jpp.krakow.pl/journal/archive/11_07_s5/pdf/591_11_07_s5_article.pdf CASE STUDY NUR 7201 1&2 Sinert R., Peacock, Jr. P.R. (2011). Chapter 91. Acute Renal Failure. In J.E. Tintinalli, J.S. Stapczynski, D.M. Cline, O.J. Ma, R.K. Cydulka, G.D. Meckler (Eds), Tintinalli's Emergency Medicine: A Comprehensive Study Guide, 7e. Retrieved from http://www.accessmedicine.com/content.aspx?aID=6361976 20