Uptravi
advertisement

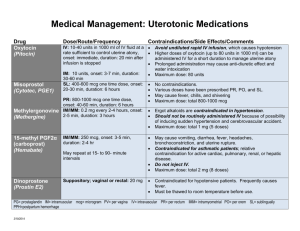
New Drug Introduction: Uptravi®/ selexipag Pharmacology Prostacyclin (IP) receptor agonist that causes vasodilation of pulmonary arteries Manufacturer Actelion Pharmaceuticals Approval Date December 21, 2015 Indications Treatment of pulmonary arterial hypertension (PAH), WHO Group I, to delay disease progression and reduce risk of hospitalization for PAH Contraindications None Black Box Warnings None Warnings & If pulmonary edema occurs, consider pulmonary veno-occlusive Precautions disease (PVOD). If PVOD confirmed, discontinue use. Pregnancy/Lactation Pregnancy: no adequate or well-controlled studies in pregnant women Lactation Recommendation: Unknown if excreted in human breast milk; recommend discontinuation of selexipag if nursing Pharmacokinetics A – Time to peak: (selexipag): 1-3 hours; (metabolite): 3-4 hours ~30% lower Cmax, and delayed time to Tmax in presence of food D – Highly bound (~99%) to plasma proteins M – Hydrolysis to yield active metabolite, then metabolism catalyzed by CYP3A4 and CYP2C8 E – Terminal t ½ (selexipag): 0.8-2.5 hours; terminal t ½ (active metabolite): 6.2-13.5 hours Drug Interactions – Avoid concomitant administration with strong inhibitors of CYP2C8 Precipitant/Object (e.g. gemfibrozil) as this results in increased exposure to selexipag and active metabolite Adverse Effects Headache (65%) [32%] Nausea (33%) [18%] (Treatment%) [Placebo%] Diarrhea (42%) [18%] Myalgia (16%) [6%] Jaw pain (26%) [6%] Vomiting (18%) [9%] Extremity pain (17%) [8%] Flushing (12%) [5%] Monitoring Efficacy 6-minute walk distance WHO functional class Dosing - Initial 200 mcg twice daily Dosing - Usual Increase dose by 200 mcg twice daily at weekly intervals to maximum tolerated dose Dosing - Max 1600 mcg twice daily Renal Adjustment No adjustment needed if eGFR > 15 mL/min/1.73 m 2 There is no clinical experience for dialysis or eGFR < 15 mL/min/1.73 m2 Hepatic Adjustment Recommend a once daily regimen for those with moderate hepatic impairment (Child-Pugh Class B) There is no clinical experience in Child-Pugh Class C impairment Avoid in severe hepatic impairment Cost: Source: Medpagetoday.com 1/6/16 Dose(s) Uptravi ® – selexipag 200, 400, 600, 800, 1000, Uptravi (selexipag) 1200, 1400, and 1600 mcg Tyvaso (orenitram) 0.125, 0.25, 1, and 2.5 mg $ (30d) or (Tx course) $13,000 per 30 days $526-$7,020 per 30 days Summary Uptravi®, selexipag, is an oral prostacyclin agonist indicated for pulmonary arterial hypertension (PAH) Selexipag should not be used with other strong CYP2C8 inhibitors Administered at 200 mcg BID and titrated on a weekly basis to the highest tolerated dose or the max dose of 1600 mcg BID Most common side effects include headache, diarrhea, jaw pain and nausea Selexipag can be used in combination with other classes of PAH medications (PDE-5 inhibitors and endothelin receptor antagonists) References: 1. www.uptravi.com 2. Uptravi package insert. Actelion. Dec. 2015. 3. Sitbon O, Channick R, Chin KM, et al. Selexipag for the Treatment of Pulmonary Arterial Hypertension. N Engl J Med. 2015;373(26):2522-33. Date Prepared: December 27, 2015 Editor: Peter G. Koval, Pharm.D., BCPS Author: Ruhaniyah Alam, Pharm.D. Candidate, UNC Eshelman School of Pharmacy