Water Properties - Needham.K12.ma.us
advertisement

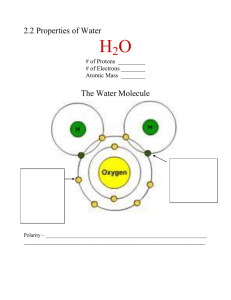
Water Properties Theme: Structure Meets Function Structure of Water • Bonds within a water molecule: polar covalent • Bonds between water molecules: hydrogen Which water property? • Watch teacher demo of ice cubes and butter cubes! Which water property? • Did you do a lab to demonstrate this property in middle school? Which water property? Which property? • Watch the teacher heat alcohol and water! Which property? Which property didn’t we see? • Example: Body’s fluids are “suspensions” and “solutions” – What types of things gs dissolve in the body’s fluids? – What types of things are “suspended”? Why can’t they dissolve? Is water really the universal solvent? • You will complete four experiments with water. • You will use your data to answer the question…like the virus question; there is not really a wrong or right answer…we are looking for you to use evidence to support your decision! • Could you design an experiment to test one of the other properties? Dissociation of water and pH • How do the structures of acids and bases change the neutral nature of pure water? • HW: Draw a pH scale in your notes; identifying values for acids, bases, and neutral & plot 3 examples (each) of acids & bases.