15gp2pp - Knockhardy
advertisement

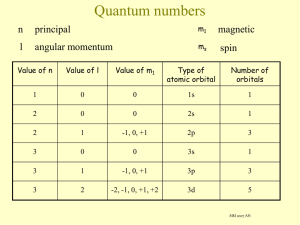
AN INTRODUCTION TO GROUP II Alkaline earths 1s2 2s2 2p6 3s2 1s2 2s2 2p6 3s23p64s2 KNOCKHARDY PUBLISHING 2015 SPECIFICATIONS KNOCKHARDY PUBLISHING GROUP II (Alkaline Earths) INTRODUCTION This Powerpoint show is one of several produced to help students understand selected topics at AS and A2 level Chemistry. It is based on the requirements of the AQA and OCR specifications but is suitable for other examination boards. Individual students may use the material at home for revision purposes or it may be used for classroom teaching with an interactive white board. Accompanying notes on this, and the full range of AS and A2 topics, are available from the KNOCKHARDY SCIENCE WEBSITE at... www.knockhardy.org.uk/sci.htm Navigation is achieved by... either clicking on the grey arrows at the foot of each page or using the left and right arrow keys on the keyboard ©HOPTON GROUP II CONTENTS • General properties • Trends in electronic configuration • Trends in atomic and ionic radius • Trends in melting point • Trends in ionisation energy • Reaction with oxygen and water • Oxides and hydroxides • Carbonates • Sulfates ©HOPTON GROUP PROPERTIES GENERAL • metals • all have the electronic configuration ... ns2 TRENDS • • • • • melting point electronic configuration electronegativity atomic size ionic size ©HOPTON THE s-BLOCK ELEMENTS Elements in Group I (alkali metals) and Group II (alkaline earths) are known as s-block elements because their valence (bonding) electrons are in s orbitals. ©HOPTON THE s-BLOCK ELEMENTS Elements in Group I (alkali metals) and Group II (alkaline earths) are known as s-block elements because their valence (bonding) electrons are in s orbitals. ALKALI METALS Gp I 1s2 2s1 1s2 2s2 2p6 3s1 1s2 2s2 2p6 3s23p64s1 Li Na K … 5s1 Rb … 6s1 Cs Fr ©HOPTON THE s-BLOCK ELEMENTS Elements in Group I (alkali metals) and Group II (alkaline earths) are known as s-block elements because their valence (bonding) electrons are in s orbitals. ALKALI METALS ALKALINE EARTHS Gp I Gp II Li Be 1s2 2s2 Na Mg 1s2 2s2 2p6 3s2 K Ca 1s2 2s2 2p6 3s23p64s2 … 5s1 Rb Sr … 6s1 Cs Ba Fr Rn 1s2 2s1 1s2 2s2 2p6 3s1 1s2 2s2 2p6 3s23p64s1 ©HOPTON … 5s2 … 6s2 THE s-BLOCK ELEMENTS Elements in Group I (alkali metals) and Group II (alkaline earths) are known as s-block elements because their valence (bonding) electrons are in s orbitals. ALKALI METALS ALKALINE EARTHS Gp I Gp II Li Be 1s2 2s2 Na Mg 1s2 2s2 2p6 3s2 K Ca 1s2 2s2 2p6 3s23p64s2 … 5s1 Rb Sr … 6s1 Cs Ba Fr Rn 1s2 2s1 1s2 2s2 2p6 3s1 1s2 2s2 2p6 3s23p64s1 Francium and radium are both short-lived radioactive elements ©HOPTON … 5s2 … 6s2 GROUP TRENDS ELECTRONIC CONFIGURATION Be Mg Ca Sr Ba Atomic Number 4 12 20 38 56 Old e/c 2,2 2,8,2 2,8,8,2 2,8,18,8,2 2,8,18,18,8,2 New e/c 1s2 2s2 …3s2 … 4s2 … 5s2 … 6s2 ©HOPTON GROUP TRENDS ELECTRONIC CONFIGURATION Be Mg Ca Sr Ba Atomic Number 4 12 20 38 56 Old e/c 2,2 2,8,2 2,8,8,2 2,8,18,8,2 2,8,18,18,8,2 New e/c 1s2 2s2 …3s2 … 4s2 … 5s2 … 6s2 As the nuclear charge increases, the electrons go into shells further from the nucleus. ©HOPTON GROUP TRENDS ELECTRONIC CONFIGURATION Be Mg Ca Sr Ba Atomic Number 4 12 20 38 56 Old e/c 2,2 2,8,2 2,8,8,2 2,8,18,8,2 2,8,18,18,8,2 New e/c 1s2 2s2 …3s2 … 4s2 … 5s2 … 6s2 As the nuclear charge increases, the electrons go into shells further from the nucleus. The extra distance of the outer shell from the nucleus affects… Atomic radius Ionisation energy Chemical reactivity Ionic radius Melting point ©HOPTON GROUP TRENDS ATOMIC & IONIC RADIUS Be Mg Ca Sr Ba Atomic radius / nm 0.106 0.140 0.174 0.191 0.198 Electronic config. 2,2 2,8,2 2,8,8,2 2,8,18,8,2 2,8,18,18,8,2 ©HOPTON GROUP TRENDS ATOMIC & IONIC RADIUS Be Mg Ca Sr Ba Atomic radius / nm 0.106 0.140 0.174 0.191 0.198 Electronic config. 2,2 2,8,2 2,8,8,2 2,8,18,8,2 2,8,18,18,8,2 ATOMIC RADIUS INCREASES down Group • the greater the atomic number the more electrons there are; these go into shells increasingly further from the nucleus ©HOPTON 1s2 2s2 2p6 3s2 1s2 2s2 2p6 3s23p64s2 GROUP TRENDS ATOMIC & IONIC RADIUS Be Mg Ca Sr Ba Atomic radius / nm 0.106 0.140 0.174 0.191 0.198 Electronic config. 2,2 2,8,2 2,8,8,2 2,8,18,8,2 2,8,18,18,8,2 ATOMIC RADIUS INCREASES down Group • the greater the atomic number the more electrons there are; these go into shells increasingly further from the nucleus 1s2 2s2 2p6 3s2 1s2 2s2 2p6 3s23p64s2 • atoms of Group II are smaller than the equivalent Group I atom the extra proton exerts a greater attraction on the electrons ©HOPTON 11 protons 1s2 2s2 2p6 3s1 12 protons 1s2 2s2 2p6 3s2 GROUP TRENDS ATOMIC & IONIC RADIUS Be Mg Ca Sr Ba Atomic radius / nm 0.106 0.140 0.174 0.191 0.198 Electronic config. 2,2 2,8,2 2,8,8,2 2,8,18,8,2 2,8,18,18,8,2 Be2+ Mg2+ Ca2+ Sr2+ Ba2+ Ionic radius / nm 0.030 0.064 0.094 0.110 0.134 Electronic config. 2 2,8 2,8,8 2,8,18,8 2,8,18,18,8 ©HOPTON GROUP TRENDS ATOMIC & IONIC RADIUS Be Mg Ca Sr Ba Atomic radius / nm 0.106 0.140 0.174 0.191 0.198 Electronic config. 2,2 2,8,2 2,8,8,2 2,8,18,8,2 2,8,18,18,8,2 Be2+ Mg2+ Ca2+ Sr2+ Ba2+ Ionic radius / nm 0.030 0.064 0.094 0.110 0.134 Electronic config. 2 2,8 2,8,8 2,8,18,8 2,8,18,18,8 IONIC RADIUS INCREASES down Group • ions are smaller than atoms – on removing the outer shell electrons, the remaining electrons are now in fewer shells ©HOPTON GROUP TRENDS ATOMIC & IONIC RADIUS Be Mg Ca Sr Ba Atomic radius / nm 0.106 0.140 0.174 0.191 0.198 Electronic config. 2,2 2,8,2 2,8,8,2 2,8,18,8,2 2,8,18,18,8,2 Be2+ Mg2+ Ca2+ Sr2+ Ba2+ Ionic radius / nm 0.030 0.064 0.094 0.110 0.134 Electronic config. 2 2,8 2,8,8 2,8,18,8 2,8,18,18,8 IONIC RADIUS INCREASES down Group • ions are smaller than atoms – on removing the outer shell electrons, the remaining electrons are now in fewer shells 1s2 2s2 2p6 3s2 1s2 2s2 2p6 ©HOPTON GROUP TRENDS ATOMIC & IONIC RADIUS Be Mg Ca Sr Ba Atomic radius / nm 0.106 0.140 0.174 0.191 0.198 Electronic config. 2,2 2,8,2 2,8,8,2 2,8,18,8,2 2,8,18,18,8,2 Be2+ Mg2+ Ca2+ Sr2+ Ba2+ Ionic radius / nm 0.030 0.064 0.094 0.110 0.134 Electronic config. 2 2,8 2,8,8 2,8,18,8 2,8,18,18,8 IONIC RADIUS INCREASES down Group • ions are smaller than atoms – on removing the outer shell electrons, the remaining electrons are now in fewer shells ©HOPTON 1s2 2s2 2p6 3s2 1s2 2s2 2p6 1s2 2s2 2p6 3s23p64s2 1s2 2s2 2p6 3s23p6 GROUP TRENDS MELTING POINT Be Mg Ca Sr Ba Melting point / ºC 1283 650 850 770 710 Electronic config. 2,2 2,8,2 2,8,8,2 2,8,18,8,2 2,8,18,18,8,2 ©HOPTON GROUP TRENDS MELTING POINT Be Mg Ca Sr Ba Melting point / ºC 1283 650 850 770 710 Electronic config. 2,2 2,8,2 2,8,8,2 2,8,18,8,2 2,8,18,18,8,2 DECREASES down Group ©HOPTON GROUP TRENDS MELTING POINT Be Mg Ca Sr Ba Melting point / ºC 1283 650 850 770 710 Electronic config. 2,2 2,8,2 2,8,8,2 2,8,18,8,2 2,8,18,18,8,2 DECREASES down Group • each atom contributes two electrons to the delocalised cloud • metallic bonding gets weaker due to increased size of ion Larger ions mean that the electron cloud doesn’t bind them as strongly ©HOPTON GROUP TRENDS MELTING POINT Be Mg Ca Sr Ba Melting point / ºC 1283 650 850 770 710 Electronic config. 2,2 2,8,2 2,8,8,2 2,8,18,8,2 2,8,18,18,8,2 DECREASES down Group • each atom contributes two electrons to the delocalised cloud • metallic bonding gets weaker due to increased size of ion Larger ions mean that the electron cloud doesn’t bind them as strongly • Group I metals have lower melting points than the equivalent Group II metal because each metal only contributes one electron to the cloud ©HOPTON GROUP TRENDS MELTING POINT ©HOPTON Be Mg Ca Sr Ba Melting point / ºC 1283 650 850 770 710 Electronic config. 2,2 2,8,2 2,8,8,2 2,8,18,8,2 2,8,18,18,8,2 DECREASES down Group • each atom contributes two electrons to the delocalised cloud • metallic bonding gets weaker due to increased size of ion Larger ions mean that the electron cloud doesn’t bind them as strongly • Group I metals have lower melting points than the equivalent Group II metal because each metal only contributes one electron to the cloud NOTE Magnesium doesn’t fit the trend because crystalline structure can also affect the melting point of a metal FIRST IONISATION ENERGY ©HOPTON FIRST IONISATION ENERGY Be Mg Ca Sr Ba 1st I.E. / kJ mol-1 899 738 590 550 500 2nd I.E. / kJ mol-1 1800 1500 1100 1100 1000 3rd I.E. / kJ mol-1 14849 7733 4912 4120 3390 ©HOPTON FIRST IONISATION ENERGY Be Mg Ca Sr Ba 1st I.E. / kJ mol-1 899 738 590 550 500 2nd I.E. / kJ mol-1 1800 1500 1100 1100 1000 3rd I.E. / kJ mol-1 14849 7733 4912 4120 3390 DECREASES down the Group Despite the increasing nuclear charge the values decrease due to the extra shielding provided by additional filled inner energy levels ©HOPTON FIRST IONISATION ENERGY Be Mg Ca Sr Ba 1st I.E. / kJ mol-1 899 738 590 550 500 2nd I.E. / kJ mol-1 1800 1500 1100 1100 1000 3rd I.E. / kJ mol-1 14849 7733 4912 4120 3390 DECREASES down the Group Despite the increasing nuclear charge the values decrease due to the extra shielding provided by additional filled inner energy levels 4+ BERYLLIUM There are 4 protons pulling on the outer shell electrons 1st I.E. = 899 kJ mol-1 ©HOPTON FIRST IONISATION ENERGY Be Mg Ca Sr Ba 1st I.E. / kJ mol-1 899 738 590 550 500 2nd I.E. / kJ mol-1 1800 1500 1100 1100 1000 3rd I.E. / kJ mol-1 14849 7733 4912 4120 3390 DECREASES down the Group Despite the increasing nuclear charge the values decrease due to the extra shielding provided by additional filled inner energy levels MAGNESIUM 4+ 12+ BERYLLIUM There are 4 protons pulling on the outer shell electrons There are now 12 protons pulling on the outer shell electrons. However, the extra filled inner shell shields the nucleus from the outer shell electrons. The effective nuclear charge is less and the electrons are easier to remove. 1st I.E. = 738 kJ mol-1 1st I.E. = 899 kJ mol-1 ©HOPTON FIRST IONISATION ENERGY ©HOPTON Be Mg Ca Sr Ba 1st I.E. / kJ mol-1 899 738 590 550 500 2nd I.E. / kJ mol-1 1800 1500 1100 1100 1000 3rd I.E. / kJ mol-1 14849 7733 4912 4120 3390 DECREASES down the Group Despite the increasing nuclear charge the values decrease due to the extra shielding provided by additional filled inner energy levels MAGNESIUM 4+ 12+ BERYLLIUM There are 4 protons pulling on the outer shell electrons There are now 12 protons pulling on the outer shell electrons. However, the extra filled inner shell shield the nucleus from the outer shell electrons. The effective nuclear charge is less and the electrons are easier to remove. 1st I.E. = 738 kJ mol-1 1st I.E. = 899 kJ mol-1 ©HOPTON SUCCESSIVE IONISATION ENERGIES Be Mg Ca Sr Ba 1st I.E. / kJ mol-1 899 738 590 550 500 2nd I.E. / kJ mol-1 1800 1500 1100 1100 1000 3rd I.E. / kJ mol-1 14849 7733 4912 4120 3390 Successive Ionisation Energy values get larger ©HOPTON SUCCESSIVE IONISATION ENERGIES Be Mg Ca Sr Ba 1st I.E. / kJ mol-1 899 738 590 550 500 2nd I.E. / kJ mol-1 1800 1500 1100 1100 1000 3rd I.E. / kJ mol-1 14849 7733 4912 4120 3390 Successive Ionisation Energy values get larger 12+ 1st I.E. = 738 kJ mol-1 ©HOPTON SUCCESSIVE IONISATION ENERGIES Be Mg Ca Sr Ba 1st I.E. / kJ mol-1 899 738 590 550 500 2nd I.E. / kJ mol-1 1800 1500 1100 1100 1000 3rd I.E. / kJ mol-1 14849 7733 4912 4120 3390 Successive Ionisation Energy values get larger 12+ 12+ 1st I.E. = 738 kJ mol-1 2nd I.E. = 1500 kJ mol-1 There are now 12 protons and only 11 electrons. The increased ratio of protons to electrons means that it is harder to pull an electron out. ©HOPTON SUCCESSIVE IONISATION ENERGIES Be Mg Ca Sr Ba 1st I.E. / kJ mol-1 899 738 590 550 500 2nd I.E. / kJ mol-1 1800 1500 1100 1100 1000 3rd I.E. / kJ mol-1 14849 7733 4912 4120 3390 Successive Ionisation Energy values get larger 12+ 12+ 1st I.E. = 738 kJ mol-1 2nd I.E. = 1500 kJ mol-1 There are now 12 protons and only 11 electrons. The increased ratio of protons to electrons means that it is harder to pull an electron out. ©HOPTON 12+ 3rd I.E. = 7733 kJ mol-1 There is a big jump in IE because the electron being removed is from a shell nearer the nucleus; there is less shielding. SUCCESSIVE IONISATION ENERGIES Be Mg Ca Sr Ba 1st I.E. / kJ mol-1 899 738 590 550 500 2nd I.E. / kJ mol-1 1800 1500 1100 1100 1000 3rd I.E. / kJ mol-1 14849 7733 4912 4120 3390 Successive Ionisation Energy values get larger 12+ 12+ 1st I.E. = 738 kJ mol-1 2nd I.E. = 1500 kJ mol-1 There are now 12 protons and only 11 electrons. The increased ratio of protons to electrons means that it is harder to pull an electron out. ©HOPTON 12+ 3rd I.E. = 7733 kJ mol-1 There is a big jump in IE because the electron being removed is from a shell nearer the nucleus; there is less shielding. CHEMICAL PROPERTIES OF THE ELEMENTS Reactivity increases down the Group due to the ease of cation formation ©HOPTON CHEMICAL PROPERTIES OF THE ELEMENTS Reactivity increases down the Group due to the ease of cation formation OXYGEN react with increasing vigour down the group Mg burns readily with a bright white flame 0 0 +2 -2 2Mg(s) + O2(g) —> 2MgO(s) Ba burns readily with an apple-green flame 2Ba(s) + O2(g) —> 2BaO(s) ©HOPTON CHEMICAL PROPERTIES OF THE ELEMENTS Reactivity increases down the Group due to the ease of cation formation OXYGEN react with increasing vigour down the group Mg burns readily with a bright white flame 0 0 +2 -2 2Mg(s) + O2(g) —> 2MgO(s) Ba burns readily with an apple-green flame 2Ba(s) + O2(g) —> 2BaO(s) In both cases… the metal is oxidised Oxidation No. increases from 0 to +2 oxygen is reduced Oxidation No. decreases from 0 to -2 O + Mg 2e¯ —> —> Mg2+ O2- ©HOPTON + 2e¯ CHEMICAL PROPERTIES OF THE ELEMENTS Reactivity increases down the Group due to the ease of cation formation ©HOPTON CHEMICAL PROPERTIES OF THE ELEMENTS Reactivity increases down the Group due to the ease of cation formation WATER react with increasing vigour down the group ©HOPTON CHEMICAL PROPERTIES OF THE ELEMENTS Reactivity increases down the Group due to the ease of cation formation WATER react with increasing vigour down the group Mg reacts very slowly with cold water Mg(s) + 2H2O(l) —> Mg(OH)2(aq) + H2(g) but reacts quickly with steam Mg(s) + H2O(g) —> MgO(s) + ©HOPTON H2(g) CHEMICAL PROPERTIES OF THE ELEMENTS Reactivity increases down the Group due to the ease of cation formation WATER react with increasing vigour down the group Mg reacts very slowly with cold water Mg(s) + 2H2O(l) —> Mg(OH)2(aq) + H2(g) but reacts quickly with steam Mg(s) + H2O(g) —> MgO(s) + Ba H2(g) reacts vigorously with cold water Ba(s) + 2H2O(l) —> Ba(OH)2(aq) + H2(g) ©HOPTON OXIDES OF GROUP II Bonding • ionic solids; EXCEPT BeO which has covalent character • BeO CaO BaO (beryllium oxide) (calcium oxide) (barium oxide) ©HOPTON MgO SrO (magnesium oxide) (strontium oxide) OXIDES OF GROUP II Bonding • ionic solids; EXCEPT BeO which has covalent character • BeO CaO BaO Reaction with water (beryllium oxide) (calcium oxide) (barium oxide) MgO SrO (magnesium oxide) (strontium oxide) BeO MgO CaO SrO BaO Reactivity with water NONE reacts reacts reacts reacts Solubility of hydroxide M(OH)2 in water Insoluble Sparingly soluble Slightly soluble Quite soluble Very soluble pH of 0.1M solution - 10.4 9-10 12.5 13.0 13.1 ©HOPTON OXIDES OF GROUP II Bonding • ionic solids; EXCEPT BeO which has covalent character • BeO CaO BaO Reaction with water (beryllium oxide) (calcium oxide) (barium oxide) MgO SrO (magnesium oxide) (strontium oxide) BeO MgO CaO SrO BaO Reactivity with water NONE reacts reacts reacts reacts Solubility of hydroxide M(OH)2 in water Insoluble Sparingly soluble Slightly soluble Quite soluble Very soluble pH of 0.1M solution - 10.4 9-10 12.5 13.0 13.1 React with water to produce the hydroxide (not Be) e.g. CaO(s) + H2O(l) —> Ca(OH)2(s) ©HOPTON HYDROXIDES OF GROUP II Properties basic strength also increases down group ©HOPTON HYDROXIDES OF GROUP II Properties • • • • • basic strength also increases down group this is because the solubility increases the metal ions get larger so charge density decreases get a lower attraction between the OH¯ ions and larger 2+ ions the ions will split away from each other more easily there will be a greater concentration of OH¯ ions in water ©HOPTON HYDROXIDES OF GROUP II Properties • • • • • basic strength also increases down group this is because the solubility increases the metal ions get larger so charge density decreases get a lower attraction between the OH¯ ions and larger 2+ ions the ions will split away from each other more easily there will be a greater concentration of OH¯ ions in water Be(OH)2 Mg(OH)2 Ca(OH)2 Sr(OH)2 Ba(OH)2 Solubility in water Insoluble Sparingly soluble Slightly soluble Quite soluble Very soluble pH of 0.1M solution - 10.4 9-10 12.5 13.0 13.1 ©HOPTON HYDROXIDES OF GROUP II Properties • • • • • basic strength also increases down group ©HOPTON this is because the solubility increases the metal ions get larger so charge density decreases get a lower attraction between the OH¯ ions and larger 2+ ions the ions will split away from each other more easily there will be a greater concentration of OH¯ ions in water Be(OH)2 Mg(OH)2 Ca(OH)2 Sr(OH)2 Ba(OH)2 Solubility in water Insoluble Sparingly soluble Slightly soluble Quite soluble Very soluble pH of 0.1M solution - 10.4 9-10 12.5 13.0 13.1 Lower charge density of the larger Ca2+ ion means that it doesn’t hold onto the OH¯ ions as strongly. More OH¯ get released into the water. It is more soluble and the solution has a larger pH. HYDROXIDES OF GROUP II Uses Ca(OH)2 used in agriculture to neutralise acid soils Ca(OH)2(s) + 2H+ (aq) —> Ca2+(aq) + 2H2O(l) Mg(OH)2 used in toothpaste and indigestion tablets as an antacid Mg(OH)2(s) + 2H+ (aq) —> Mg2+(aq) + 2H2O(l) Both the above are weak alkalis and not as caustic as sodium hydroxide ©HOPTON CARBONATES OF GROUP II ©HOPTON CARBONATES OF GROUP II Properties • insoluble in water Solubility g/100cm3 of water MgCO3 CaCO3 SrCO3 BaCO3 1.5 x 10-4 1.3 x 10-5 7.4 x 10-6 9.1 x 10-6 ©HOPTON CARBONATES OF GROUP II Properties • insoluble in water MgCO3 CaCO3 SrCO3 BaCO3 Solubility g/100cm3 of water 1.5 x 10-4 1.3 x 10-5 7.4 x 10-6 9.1 x 10-6 Decomposition temperature / ºC 400 980 1280 1360 • undergo thermal decomposition to oxide and carbon dioxide e.g. MgCO3(s) —> MgO(s) + CO2(g) ©HOPTON CARBONATES OF GROUP II Properties • insoluble in water MgCO3 CaCO3 SrCO3 BaCO3 Solubility g/100cm3 of water 1.5 x 10-4 1.3 x 10-5 7.4 x 10-6 9.1 x 10-6 Decomposition temperature / ºC 400 980 1280 1360 • undergo thermal decomposition to oxide and carbon dioxide e.g. MgCO3(s) —> MgO(s) + CO2(g) • the ease of decomposition decreases down the group ©HOPTON CARBONATES OF GROUP II Properties • insoluble in water MgCO3 CaCO3 SrCO3 BaCO3 Solubility g/100cm3 of water 1.5 x 10-4 1.3 x 10-5 7.4 x 10-6 9.1 x 10-6 Decomposition temperature / ºC 400 980 1280 1360 • undergo thermal decomposition to oxide and carbon dioxide e.g. MgCO3(s) —> MgO(s) + CO2(g) • the ease of decomposition decreases down the group EASIER HARDER ©HOPTON CARBONATES OF GROUP II Properties • insoluble in water MgCO3 CaCO3 SrCO3 BaCO3 Solubility g/100cm3 of water 1.5 x 10-4 1.3 x 10-5 7.4 x 10-6 9.1 x 10-6 Decomposition temperature / ºC 400 980 1280 1360 • undergo thermal decomposition to oxide and carbon dioxide e.g. MgCO3(s) —> MgO(s) + CO2(g) • the ease of decomposition decreases down the group EASIER HARDER One might think that the greater charge density of the smaller Mg2+ would mean that it would hold onto the CO32- ion more and the ions would be more difficult to separate. ©HOPTON CARBONATES OF GROUP II ©HOPTON Properties • insoluble in water MgCO3 CaCO3 SrCO3 BaCO3 Solubility g/100cm3 of water 1.5 x 10-4 1.3 x 10-5 7.4 x 10-6 9.1 x 10-6 Decomposition temperature / ºC 400 980 1280 1360 • undergo thermal decomposition to oxide and carbon dioxide e.g. MgCO3(s) —> MgO(s) + CO2(g) • the ease of decomposition decreases down the group EASIER HARDER One might think that the greater charge density of the smaller Mg2+ would mean that it would hold onto the CO32- ion more and the ions would be more difficult to separate. The driving force must be the formation of the oxide. The smaller ion with its greater charge density holds onto the O2- ion to make a more stable compound. GROUP TRENDS SULFATES Solubility g/100cm3 of water MgSO4 CaSO4 SrSO4 BaSO4 3.6 x 10-1 1.1 x 10-3 6.2 x 10-5 9.0 x 10-7 ©HOPTON GROUP TRENDS SULFATES Solubility g/100cm3 of water MgSO4 CaSO4 SrSO4 BaSO4 3.6 x 10-1 1.1 x 10-3 6.2 x 10-5 9.0 x 10-7 SOLUBILITY DECREASES down the Group • as the cation gets larger it has a lower charge density • it becomes less attracted to the polar water molecules ©HOPTON GROUP TRENDS SULFATES Solubility g/100cm3 of water MgSO4 CaSO4 SrSO4 BaSO4 3.6 x 10-1 1.1 x 10-3 6.2 x 10-5 9.0 x 10-7 SOLUBILITY DECREASES down the Group • as the cation gets larger it has a lower charge density • it becomes less attracted to the polar water molecules Greater charge density of Mg2+ ion means that it is more attracted to water so the ionic lattice breaks up more easily ©HOPTON GROUP TRENDS SULFATES Solubility g/100cm3 of water MgSO4 CaSO4 SrSO4 BaSO4 3.6 x 10-1 1.1 x 10-3 6.2 x 10-5 9.0 x 10-7 SOLUBILITY DECREASES down the Group • as the cation gets larger it has a lower charge density • it becomes less attracted to the polar water molecules Greater charge density of Mg2+ ion means that it is more attracted to water so the ionic lattice breaks up more easily Lower charge density of larger Ca2+ means that it is less attracted to water so the ionic lattice breaks up less easily – IT IS LESS SOLUBLE ©HOPTON GROUP TRENDS SULFATES Solubility g/100cm3 of water MgSO4 CaSO4 SrSO4 BaSO4 3.6 x 10-1 1.1 x 10-3 6.2 x 10-5 9.0 x 10-7 SOLUBILITY DECREASES down the Group • as the cation gets larger it has a lower charge density • it becomes less attracted to the polar water molecules Greater charge density of Mg2+ ion means that it is more attracted to water so the ionic lattice breaks up more easily USE Lower charge density of larger Ca2+ means that it is less attracted to water so the ionic lattice breaks up less easily – IT IS LESS SOLUBLE barium sulfate’s insolubility is used as a test for sulfates ©HOPTON AN INTRODUCTION TO GROUP II Alkaline earths THE END ©HOPTON ©2015 JONATHAN HOPTON & KNOCKHARDY PUBLISHING