Title and Source: Solid Water - http://www.elmhurst.edu/~chm/demos
advertisement

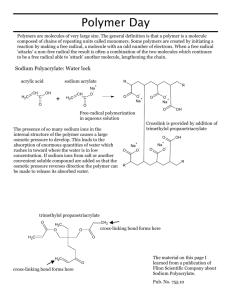
Title and Source: Solid Water - http://www.elmhurst.edu/~chm/demos/ Materials: Two 400mL beakers Water Table salt Sodium polyacrylate – may be acquired from chemical supplier in pure form or may be substituted with stuffing found in baby diapers Food colouring Stirring rod Weighing cups/ trays Weigh scale Scoopula Safety: consult sodium polyacrylate MSDS – do not allow ingestion of chemical or contact with eyes, and avoid prolonged contact with skin. Sodium polyacrylate is slippery when wet – avoid spills and ensure sufficient clean-up should they occur. Food colouring may stain surfaces, clothing, or skin upon contact. Set-Up: Measure ~300mL water into one beaker – add a few drops food colouring Measure 5 – 7 grams sodium polyacrylate into second beaker Quickly pour water into second beaker with sodium polyacrylate – pour from height of ~12 inches to ensure vigorous mixing (stirring with rod after addition of water does not achieve wanted results) What has happened to the mixture? (Turn beaker upside down to demonstrate) Measure ~10 grams table salt into weighing cup Pour salt onto the solid water and mix with stirring rod until becomes liquid again – more table salt may be needed to acquire liquid consistency, add as needed Now, what has happened to the mixture? (Pour into other beaker to demonstrate) Clean-Up: Liquid mixture – rinse down sink drain with plenty of water Solid mixture – dispose of in garbage Wash beakers and stirring rod with water (and soap if needed) Wipe down demo area with water and paper towel or cloth Wash hands with soap and water What Will Happen: Quick, vigorous mixing of the water and sodium polyacrylate causes a gelling of the water, making it into a “solid”. Addition of salt to the gel and stirring restores the water to liquid form. Why It Happens: Sodium polyacrylate is made of sodium ions surrounded by a polymer membrane. When water is mixed with sodium polyacrylate, the area inside the polymer membrane is more concentrated with ions than the area outside creating a concentration gradient. Through the process of osmosis, water passes through the polymer from the area of low ion concentration to area of high ion concentration. This movement of water into the polyacrylate causes the compound “cells” to swell and form a gel of solid water. The addition of table salt results in a reversal of the concentration gradient. Increasing the ion concentration outside the polymer membrane to greater than the concentration inside causes the water to pass back through the polymer, freeing it back into liquid form. http://www.elmhurst.edu/~chm/demos/ Bassam Z. Shakhashiri. (1989). CHEMICAL DEMONSTRATIONS: A Handbook for Teachers of Chemistry. Volume 3: 368 – 371. The University of Wisconsin Press, 1930 Monroe Street, 3rd Floor, Madison, Wisconsin 53704 Brooker, Robert J, et al. (2010). Biology, Canadian Edition. McGraw – Hill Ryerson Limited. pg. 134 – 137. Karp, Gerald. (2010). Cell and Molecular Biology: Concepts and Experiments. John Wiley & Sons Inc. pg. 144 – 145. Curriculum Fit: Science 8 (SK) – Life Science: Cells, Tissues, Organs, and Systems (CS8.1) Analyze the characteristics of cells, and compare structural and functional characteristics of plant and animal cells. [SI] Biology 30 (SK) – Cell Structure and Function 1. Describe the structures and functions of cell components. 2. Explain how the processes of diffusion, active transport, photosynthesis, and respiration are accomplished in a cell. Wellness 10 (SK) – W11 Make informed decisions regarding personal healthy eating practices based on connections to wellness. Grade 8 Science (AB) – Unit B: Cells and Systems 2. Investigate and describe the role of cells within living things 3. Interpret the healthy function of human body systems, and illustrate ways the body reacts to internal and external stimuli Grade 8 Science (MB) – Cluster 1: Cells and Systems (8-1-01) Use appropriate vocabulary related to their investigations of cells and systems. Include: cell theory, osmosis, diffusion, selective permeability, unicellular, multicellular, specialized cells and tissues, organs, systems, arteries, veins, capillaries, terms related to cell structure, heart structure, components of blood, and primary and secondary defense systems. GLO: C6, D1 Grade 8 Science (BC) – Life Science: Cells and Systems (B2) Relate the main features and properties of cells to their functions