A.P. Chemistry

A.P. Chemistry
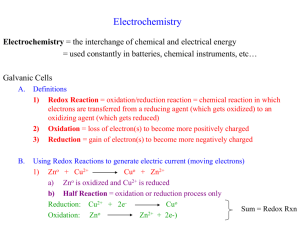
Electrochemistry
Electrochemistry - study of the interchange of chemical & electrical energy (p. 837)
(Review Ch. 4. Aqueous Chemistry: Redox; ½ Reaction Writing;
Determining Oxidatn #)
Oxidation- Reduction Reactions (p. 838)- a transfer of electrons from a reducing agent to an oxidizing agent.
Oxidation is Loss of electrons
Reduction is Gain of Electrons
(OIL RIG)
Writing ½ Reactions
Example: 2Na(s) + Cl
2
(g) 2NaCl (S)
Oxidation: 2Na 2Na+ + 2e-
Reduction: Cl
2
+ 2e
Example: 8H + (aq) + MnO
4
-
2Cl-
(aq) + 5Fe +2 (aq) Mn +2 (aq) +
5Fe +3 (aq) + 4H
2
O(l)
Oxidation:
Reduction:
Galvanic Cell- a device in which chemical energy is changed to electrical energy (p. 839) Draw it!
Anode- electrode compartment where oxidation occurs (p.
839)(vowels)
Cathode- electrode compartment where reduction occurs (p.
839)(consonants)
Cell Potential (E cell
) or electromotive force (emf)- the “pull” or driving force on the electrons in a galvanic cell.
Volt- unit of electrical potential
1 V = 1 Joule of work per 1 coulomb of charge transfer
Ampere- the unit of electric current equal to one coulomb of charge per second
1 A = 1C/s
Charge on an electron- 1.60218 x 10 -19 C
(coulombs)
Faraday- 1 F = 96485 Coulombs/mol
(on equation sheet, rounded to 96,500)
Electrode Potentials and Half-Cells (p. 840-845)
When a metal comes into contact with a solution containing its own ions, an equilibrium is set up.
M x+ (aq) + xe M(s)
Some reactive metals such as Mg will lose electrons readily and the equilibrium lies to the left. A large number of electrons are released which collect on the surface of the metal giving a negative charge.
Mg +2 (aq) + 2e Mg(s)
A less reactive metal such as silver will show less tendency to ionize and the equilibrium lies to the right. Fewer electrons will collect on the metal and the charge will be much less negative. In fact, if the aqueous ions remove electrons from the metal, it will develop a positive charge.
Ag + (aq) + e Ag(s)
Non-metals can also be considered, for ex.,
H + (aq) + e ½ H
2
(g)
Whenever an element is placed in contact with a solution containing its own ions, an electric charge will develop on the metal or, in the case of a nonmetal, on the inert conductor placed in solution. The charge is called the electrode potential and the system is called a half-cell. The sign and size of the charge will depend on the ability of the element to lose or gain electrons. See the Table of
Standard Reduction Electrode Potentials (SREP) (on the back of the Periodic Table) or p. 843 in your textbook.
Standard potentials are given as reduction processes. Two manipulations are necessary:
One must be reversed in the calculations (reverse the sign, too)
#electrons lost must equal the # electrons gained. Therefore, multiply equation by the appropriate integer.
(The E o cell is not changed and is not multiplied by the integer required to balance the cell
equation (it is an intensive property). (p. 844)
Zn(s) + Cu 2+ (aq) Cu(s) + Zn 2+ (aq)
Anode: Zn Zn 2+ + 2e-
Cathode: Cu 2+ + 2e Cu
E o cell = E o
Zn Zn2+
(E o = E o ox
+ E o red
)
1.10 V = 0.76 + ?
+ E o
Cu2+ Cu
? = 0.34 V
Example: Consider a galvanic cell based on the following reaction:
Al +3 (aq) + Mg(s) Al(s) + Mg +2 (aq)
Write the ½ reactions:
Find the E o (standard electrode potential values) values: (look on p. 843)
Give the balanced cell reaction and determine E o for the cell.
Example: A galvanic cell is based on the following reaction:
MnO
4
(aq) + H + (aq) + ClO
Mn +2 (aq) + H
2
O(aq)
3
(aq) ClO
4
(aq) +
The half-reactions are:
MnO
4
4H
2
O
ClO
4
(aq) + 5e- + 8H + (aq) Mn +2 (aq) +
E o = 1.51V
(aq) + 2H + (aq) + 2e ClO
E o = 1.19V
3
(aq) + H
2
O
Give the balanced cell reaction and determine the value of E o for the cell.
Electrochemical Species and Electrode Potentials
(Look at the review sheet: SRP table)
Elements that appear at the top of the electrochemical series lose electrons most readily and therefore have the most negative E o values and are the best reducing agents. (You can derive chemical activity from this.)
Elements that appear at the bottom of the series gain electrons most readily and therefore have the most positive E o values and are the best oxidizing agents.
Electrochemical Cells
An electrochemical cell is the apparatus for generating electrical energy from a spontaneous REDOX reaction.
Connecting two half-cells that have different electrode potentials will form an electrochemical cell.
A high resistance voltmeter is used to measure the voltage since it draws no current and will not affect the reading.
A salt bridge connects the two half-cells. It can be made from a piece of filter paper soaked in a suitable ionic solution, often potassium chloride or potassium nitrate. The ionic solution used in the salt bridge must not interfere with the equilibrium of the two half-cells; for ex., KCl could not be used if one of the half-cells contained Ag + ions as they would react with the Cl ions, precipitating the silver out and reducing the concentration of Ag + ions. The salt bridge allows the transfer of ions between the two solutions, thus completing the circuit. Ions will flow in order to balance the charge.
Measurement of Electromotive Force (EMF) or E cell
The EMF or E cell is the voltage measured when no current is being drawn from the cell and is determined using a high resistance voltmeter. It can be calculated by applying the following relationship to the cell diagram.
E cell
= E
RHS
- E
LHS
(same thing as E ox
+ E red
, once you reverse the equation from the standard reduction potential chart and change the sign)
Since the half reactions are reversible they are subject to change according to Le Chatelier’s Principle. In order to make more meaningful comparisons, it is necessary to stipulate a set of standard conditions under which the electrode potential of a given half-cell is measured.
Standard Electrode Potential (SHE)
The Standard Electrode Potential of a half-cell,
E o cell
, is defined as the electrode potential of a half-cell, measured relative to a standard hydrogen electrode which has a value of
0.00 V, measured under standard conditions
(temperature @ 25 o C (298 K), gases at 1 atm, all solutions at 1 M concentration). The diagram below shows the standard hydrogen electrode being used to determine the E o for an electrode being tested.
The practical use of the hydrogen half-cell for determining E o values suffers from three main problems.
• It is difficult to set up the H
2
(g) at precisely
1 atm pressure.
• It is fragile and non-portable.
• The equilibrium H + (aq) + e ½ H
2
(g) is only established slowly.
Example:
Fe 3+ (aq) + Cu(s) Cu 2+ (aq) + Fe 2+ (aq)
Fe 3+ + e Fe 2+ E o = 0.77 V
Cu 2+ + 2e Cu E o = 0.34 V
*reverse, therefore,
Cu Cu 2+ + 2e-
Multiply equation #1
2Fe 3+ + 2e 2Fe 2+ E o = 0.77 V
(NO CHANGE!)
(Now, add them together)
E o = -0.34 V + 0.77 V =
Cell diagrams have a number of standard features. The reduced species is always placed on the RHS and this electrode is the cathode. The oxidized species is always placed on the LHS and this electrode is the anode. (Watch
out: some AP questions have reversed the cell diagrams- you must be able to identify which is the anode and which is the cathode based on the processes occurring.)
The vertical line │ represents the different phases present in each electrode.
The double vertical line ║ represents the salt bridge connecting the two electrodes.
A(electrode) │ A + (solution) ║ C + (solution) │ C(electrode)
Different species in the same phase are separated by a comma, for ex., Fe +2
(aq), Fe +3 (aq). The order in which they are written is not important.
The presence of an inert conductor (required when no solid metal is present) may also be shown inside parentheses ( ). For ex., (Pt) ½ H
2
(g) , H + (aq)
The electrons will flow toward the more positive half-cell, in this case, from zinc to copper. As a result, the copper ions gain electrons and are therefore reduced. A “cell diagram” can be written thus
Zn(s) │Zn +2 (aq) ║ Cu +2 (aq) │Cu(s)
Example: (p. 846)
Fe(s) │ Fe 2+ (aq) ║ MnO
4
(aq), Mn +2 (aq) │ Pt(s)
Problem: Describe completely the galvanic cell based on the following half-reactions under standard conditions:
Ag
Fe
+
+3
+ e Ag
+ e Fe +2
E
E o o
= 0.80 V
= 0.77 V
1. must have E o cell reverse.
positive; therefore, 2 nd reaction must run in
2. Ag + receives electrons, Fe containing Ag +
+2 ion loses electrons, electrons will flow from the compartment containing Fe +2 to the compartment
3. Oxidation occurs in the Fe +2 compartment (the anode)
4. Must use an inert electrode in the Fe +2
Therefore, line notation is:
/Fe +3 compartment.
The Relationship between Gibbs Free Energy and E cell
The relationship between Gibbs Free Energy and the E is summarized by the expression cell
/\G o = -n F E o cell
Where
F = Faraday constant = 96500 J/V mol n = number of moles of electrons transferred
Example: Calculate the standard free energy for the zinc-silver galvanic cell.
Coupling this with the expression below (see Unit
13)
/\G o = -RT ln K
R is the ideal gas constant; there are several different values of R, depending on the equation you are using and the units of the other quantities in the equation; for electrochemistry,
R = 8.314 J/K mol
Example: Calculate the equilibrium constant for the zinc-silver cell in the previous example.
It is fairly simple to derive the expression
E o cell
= RT ln K nF
At T = 298 K, and substituting for R and F, the expression can be simplified to
E o cell
= 0.0257 ln K n
The Nernst Equation and Non-standard conditions of temperature and concentration
The Nernst equation can be used to calculate the voltage generated by the combination of two half-cells when the conditions are not standard. One form of the equation is
E = E o cell
- RT lnQ nF
Where R is the gas constant (8.314 J/K mol), T is the Kelvin temperature, n is the # of electrons transferred between the species, F is the Faraday constant, E o cell is the voltage generated IF the conditions WERE standard, ln is the natural logarithm, and Q is the reaction quotient.
(To review logarithm and natural logarithm, read p. A4-A6 in the textbook)
(The reaction quotient is defined as the concentrations of the product ions, raised to their stoichiometric powers and multiplied together, divided by the concentrations of the reactant ions, raised to their stoichiometric powers and multiplied together. Any pure solids or liquids are omitted from the reaction quotient. See Unit 13)
This equation allows us to calculate the cell voltage at any concentration of reactants and products and at any temperature. Commonly it is only the concentrations of ions that have changed, and at 298 K, the expression can be simplified to
(Nernst eqx.)
E = E o cell
- 0.0257 lnQ = n
E o cell
– 0.0592 V log Q n
Example: Determine the measured emf
(electromotive force) for the zinc-silver cell in the previous example if the concentration of the zinc in the zinc half-cell is 1.10 M and the concentration of the silver in the silver halfcell is 0.09 M.
Example: The Danielle cell composed of a zinc half-cell and a copper half-cell has a measured voltage of 1.25 V. The standard cell potential is
1.10 V. If the concentration of the copper halfcell is 0.60 M, what is the concentration of the zinc half-cell?
As cell voltage (E) declines with reactants converting to products, E eventually reaches zero. Zero potential means the reaction is at equilibrium.
If the cell is at equilibrium, E o cell
Q must equal K,
= 0, and then, logK = n E o
0.0592
Example:
If the reaction below is carried out using solutions that 5 M [Zn +2 ] and 0.3 M [Cu +2 ] at
298 K, what is the actual cell voltage?
Zn(s) + Cu 2+ (aq) Cu(s) + Zn +2 (aq)
First, work out the E
Zn(s) │ Zn o
+2 cell assuming standard conditions
(aq) ║ Cu +2 (aq) │ Cu(s)
Zn +2 (aq) + 2e Zn(s)
Cu +2 (aq) + 2e Cu(s)
E
E o o
= -0.76 V
= +0.34 V
E o cell
= E
R
- E
L
= +0.34 - (-0.76) = 1.1 V
Then, calculate Q. Since zinc and copper metals are solids, they are omitted from Q.
Q = [Zn +2 ] = 5 = 16.7
[Cu +2 ] 0.3
Two electrons are transferred between the zinc and the copper, so n = 2. Plug everything in
E = 1.1 V 0.0257 ln(16.7) = 1.06 V
2
Electrolysis
Electrolysis is the process in which electrical energy is used to cause a non-spontaneous
REDOX reaction to occur. It is the opposite of an electrochemical cell. An electrolytic cell is one that is used to carry out electrolysis.
Anions are attracted towards the anode where they undergo a process of oxidation. Electrons flow from the anode to the cathode where cations undergo a process of reduction.
Quantitative Aspects of Electrolysis
The amount of a substance produced in an electrolytic cell can be calculated using Faraday’s Law.
Calculate the number of Faradays passed in the electrolysis by using the expression
Number of Faradays = Current (in amps) x Time (in seconds)
96500
Then use the stoichiometry of the electrode process to determine the mass of product deposited at the electrode.
Remembering that a process that produces one mole of a product by the transfer of one electron will require one
Faraday to produce that one mole, a process that produces one mole of product by the transfer of two electrons will require two Faradays to produce that one mole, etc.
Current = charge/time = coulombs/seconds = amps
Problem: a car battery is listed as 675 “cold cranking amps,” which means that it can produce 675 amps for 30 seconds. If the halfreaction is
Pb(s) + SO
4
2 PbSO
4
+ 2e-
Then how much lead is consumed in the 30 seconds?
Problem: An aqueous copper (II) chloride solution is electrolyzed for a period of 156 minutes using a current of 9.00 A. If inert electrodes are used in the process, how many grams of copper were removed from the solution?
Problem: How long, in hours, does it take to remove all of the polonium from an aqueous
PoCl
4 solution that contains 1958 g of PoCl using a current of 6.80 A?
4
Example: Calculate the mass of bromine liquid produced in the electrolysis of an aqueous solution of sodium bromide if a current of
2.50 A is passed through the solution for 3.0 hr.
Example: A 1.00 M aqueous solution of chromium (III) bromide will be electrolyzed to
“chrome plate” a faucet. How many hours will it take to deposit 75.0 g of chromium metal from the solution when a current of 3.00 A is running through the solution?