Chapter 3: The Molecules of Cells
advertisement

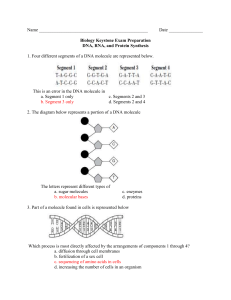
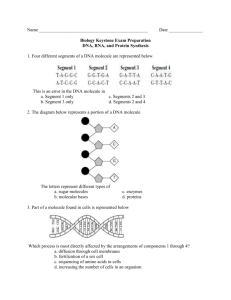
Chapter 3 The Molecules of Cells Organic Chemistry: Carbon Based Compounds A. Inorganic Compounds: Compounds without carbon. B. Organic Compounds: Compounds synthesized by cells and containing carbon (except for CO and CO2). Diverse group: Several million organic compounds are known and more are identified every day. Common: After water, organic compounds are the most common substances in cells. Over 98% of the dry weight of living cells is made up of organic compounds. Less than 2% of the dry weight of living cells is made up of inorganic compounds. Carbon: unique element for basic building block of molecules of life Carbon has 4 valence electrons: Can form four covalent bonds Can form single , double, triple bonds. Can form large, complex, branching molecules and rings. Carbon atoms easily bond to C, N, O, H, P, S. Huge variety of molecules can be formed based on simple bonding rules of basic chemistry Organic Compounds are Carbon Based Carbon Can Form 4 Covalent Bonds Different Carbon Skeletons of Organic Compounds Diversity of Organic Compounds Hydrocarbons: Organic molecules that contain C and H only. Good fuels, but not biologically important. Undergo combustion (burn in presence of oxygen). In general they are chemically stable. Nonpolar: Do not dissolve in water (Hydrophobic). Examples: (1C) Methane: CH4 (Natural gas). (2C) Ethane: CH3CH3 (3C) Propane: CH3CH2CH3 (Gas grills). (4C) Butane: CH3CH2CH2CH3 (Lighters). (5C) Pentane: CH3CH2CH2CH2CH3 (6C) Hexane: CH3CH2CH2CH2CH2CH3 (7C) Heptane: CH3CH2CH2CH2CH2CH2CH3 (8C) Octane: CH3CH2CH2CH2CH2CH2CH2CH3 Hydrocarbons have C and H only Isomers: Compounds with same chemical formula but different structure (arrangement of atoms) Isomers have different physical and chemical properties Structural Isomers: Differ in bonding arrangements Butane (C4H10) CH3--CH2--CH2--CH3 Number Isobutane (C4H10) CH3 | CH3---CH---CH3 of possible isomers increases with increasing number of carbon atoms. Functional groups play pivotal role in chemical & physical properties of organic molecules Compounds that are made up solely of carbon and hydrogen are not very reactive. Functional groups: One or more H atoms of the carbon skeleton may be replaced by a functional group. Groups of atoms that have unique chemical and physical properties. Usually a part of molecule that is chemically active. Similar activity from one molecule to another. Together with size and shape, determine unique bonding and chemical activity of organic molecules. Functional Groups Determine Chemical & Physical Properties of Organic Molecules Four Important Functional Groups: Hydroxyl (-OH) Carbonyl (=C=O) Carboxyl (-COOH) Amino Notice polar. (-NH2) that all four functional groups are A. Hydroxyl Group (-OH) Is a polar group: Polar covalent bond between O and H. Can form hydrogen bonds with other polar groups. Generally makes molecule water soluble. Example: Alcohols: Organic molecules with a simple hydroxyl group: Methanol (wood alcohol, toxic) Ethanol (drinking alcohol) Propanol (rubbing alcohol) B. Carbonyl Group (=CO) Is a polar group: O can be involved in H-bonding. Generally makes molecule water soluble. Examples: Aldehydes: Carbonyl is located at end of molecule Ketone: Carbonyl is located in middle of molecule Examples: Sugars (Aldehydes or ketones) Formaldehyde (Aldehyde) Acetone (Ketone) Sugars Have Both -OH and =CO Functional Groups C. Carboxyl Group (-COOH) Is a polar group Generally Acidic makes molecule water soluble because it can donate H+ in solution Example: Carboxylic acids: Organic acids, can increase acidity of a solution: Acetic acid: Sour taste of vinegar. Ascorbic acid (Vitamin C): Found in fruits and vegetables. Amino acids: Building blocks of proteins. D. Amino Group (-NH2) Is a polar group Generally Weak makes molecule water soluble base because N can accept a H+ Amine -general term given to compound with (-NH2) Example: Amino acids: Building blocks of proteins. Amino acid Structure: Central carbon with: H atom Carboxyl group Amino group Variable R-group Amino Acid Structure: H | (Amino Group) NH2---C---COOH (Carboxyl group) | R (Varies for each amino acid) Amino Acids Have Both -NH2 and -COOH Groups The Macromolecules of Life: Carbohydrates, Proteins, Lipids, and Nucleic Acids I. Most Biological Macromolecules are Polymers Polymer: Large molecule consisting of many identical or similar “subunits” linked through covalent bonds. Monomer: “Subunit” or building block of a polymer. Macromolecule: Large organic polymer. Most macromolecules are constructed from about 70 simple monomers. Only about 70 monomers are used by all living things on earth to construct a huge variety of molecules Structural variation of macromolecules is the basis for the enormous diversity of life on earth. Relatively few monomers are used by cells to make a huge variety of macromolecules Macromolecule Monomers or Subunits 1. Carbohydrates 20-30 monosaccharides or simple sugars 2. Proteins 20 amino acids 3. Nucleic acids (DNA/RNA) 4 nucleotides (A,G,C,T/U) 4. Lipids (fats and oils) ~ 20 different fatty acids and glycerol. Making and Breaking Polymers There are two main chemical mechanisms in the production and break down of macromolecules. Condensation or Dehydration Synthesis Hydrolysis In the cell these mechanisms are regulated by enzymes. Making Polymers A. Condensation or Dehydration Synthesis reactions: Synthetic process in which a monomer is covalently linked to another monomer. The equivalent of a water molecule is removed. General Reaction: X - OH + HO - Y --------> Monomer 1 Monomer 2 (Unlinked) Anabolic (or Polymer) X - O - Y + H2 O Dimer Water (or Polymer) Reactions: Used by cells to make large molecules from smaller ones. Require energy (endergonic) Require catalysis by enzymes Condensation Synthesis: Monomers are Linked and Water is Removed Breaking Polymers B. Hydrolysis Reactions: “Break with water”. Degradation of polymers into component monomers. Involves breaking covalent bonds between subunits. Covalent bonds are broken by adding water. General Reaction: X - O - Y + H2O ----------> X - OH + HO - Y Polymer Water Monomer 1 Monomer 2 (or Dimer) Catabolic Reactions: Used by cells to break large molecules into smaller ones. Release energy (exergonic) Reactions catalyzed by enzymes Hydrolysis: Polymers are Broken Down as Water is Added Hydrolysis Making and Breaking Polymers Examples: Dehydration Synthesis (Condensation): Enzyme Glucose + Fructose ---------> Sucrose (Monomer) (Monomer) (Dimer) + H2O Water Hydrolysis: Sucrose (Dimer) + Enzyme H2O ---------> Glucose + Fructose Water (Monomer) (Monomer) Synthesis and Hydrolysis of Sucrose III. Carbohydrates: Molecules that store energy and are used as building materials General Simple Formula: (CH2O)n sugars and their polymers. Diverse group includes sugars, starches, cellulose. Biological Functions: • Fuels, energy storage • Structural component (cell walls) • DNA/RNA component Three types of carbohydrates: A. Monosaccharides B. Disaccharides C. Polysaccharides A. Monosaccharides: “Mono” single & “sacchar” sugar Preferred source of chemical energy for cells (glucose) Can be synthesized by plants from light, H2O and CO2. Store energy in chemical bonds. Carbon skeletons used to synthesize other molecules. Characteristics: 1. May have 3-8 carbons. -OH on each carbon; one with C=0 2. Names end in -ose. Based on number of carbons: 5 carbon sugar: pentose 6 carbon sugar: hexose. 3. Can exist in linear or ring forms 4. Isomers: Many molecules with the same molecular formula, but different atomic arrangement. Example: Glucose and fructose are both C6H12O6. Fructose is sweeter than glucose. Monosaccharides Can Have 3 to 8 Carbons Linear and Ring Forms of Glucose B. Disaccharides: “Di” double & “sacchar” sugar Covalent bond formed by condensation reaction between 2 monosaccharides. Examples: 1. Maltose: Glucose + Glucose. • Energy storage in seeds. • Used to make beer. 2. Lactose: Glucose + Galactose. • Found in milk. • Lactose intolerance is common among adults. • May cause gas, cramping, bloating, diarrhea, etc. 3. Sucrose: Glucose + Fructose. • Most common disaccharide (table sugar). • Found in plant sap. Maltose and Sucrose are Disaccharides C. Polysaccharides: “Poly” many (8 to 1000) Functions: Storage of chemical energy and structure. Storage polysaccharides: Cells can store simple sugars in polysacharides and hydrolyze them when needed. 1. Starch: Glucose polymer (Helical) Form of glucose storage in plants (amylose) Stored in plant cell organelles called plastids 2. Glycogen: Glucose polymer (Branched) Form of glucose storage in animals (muscle and liver cells) Three Different Polysaccharides of Glucose Structural Polysaccharides: Used as structural components of cells and tissues. 1. Cellulose: Glucose polymer. The major component of plant cell walls. CANNOT be digested by animal enzymes. Only microbes have enzymes to hydrolyze. 2. Chitin: Polymer of an amino sugar (with NH2 group) Forms exoskeleton of arthropods (insects) Found in cell walls of some fungi Cellulose: Polysaccharide Found in Plant and Algae Cell Walls Proteins: Large three-dimensional macromolecules responsible for most cellular functions Polypeptide chains: Polymers of amino acids linked by peptide bonds in a SPECIFIC linear sequence Protein: Macromolecule composed of one or more polypeptide chains folded into SPECIFIC 3-D conformations Proteins have important and varied functions: 1. Enzymes: Catalysis of cellular reactions 2. Structural Proteins: Maintain cell shape 3. Transport: Transport in cells/bodies (e.g. hemoglobin). Channels and carriers across cell membrane. 4. Communication: Chemical messengers, hormones, and receptors. 5. Defensive: Antibodies and other molecules that bind to foreign molecules and help destroy them. 6. Contractile: Muscular movement. 7. Storage: Store amino acids for later use (e.g. egg white). Protein function is dependent upon its 3-D shape. Polypeptide: Polymer of amino acids connected in a specific sequence A. Amino acid: The monomer of polypeptides Central carbon • H atom • Carboxyl group • Amino group • Variable R-group Protein Function is dependent upon Protein Structure (Conformation) CONFORMATION: The 3-D shape of a protein is determined by its amino acid sequence. Four Levels of Protein Structure 1. Primary structure: Linear amino acid sequence, determined by gene for that protein. 2. Secondary structure: Regular coiling/folding of polypeptide. Alpha helix or beta sheet. Caused by H-bonds between amino acids. Primary Structure of Protein: Amino Acid Sequence is Determined by Gene Secondary Structure of Protein: Regular Folding Patterns (Alpha Helix or Pleated Sheet) 3. Tertiary structure: Overall 3-D shape of a polypeptide chain. 4. Quaternary structure: Only in proteins with 2 or more polypeptides. Overall 3-D shape of all chains. Example: Hemoglobin (2 alpha and 2 beta polypeptides) Tertiary Structure: Overall 3-D Shape of Protein Tertiary Structure of Lysozyme Quaternary Structure: Overall 3-D Shape of Protein with 2 or More Subunits What determines a protein’s shape? A. Primary structure: Exact location of each amino acid along the chain determines the protein’s folding pattern. Example: Sickle Cell Hemoglobin protein Mutation changes amino acid #6 on the alpha chain. Defective hemoglobin causes red blood cells to assume sickle shape, which damages tissue and capillaries. Sickle cell anemia gene is carried in 10% of African Americans. B. Chemical & Physical Environment: Presence of other compounds, pH, temperature, salts. Denaturation: Process which alters native conformation and therefore biological activity of a protein. Several factors can denature proteins: pH and salts: Disrupt hydrogen, ionic bonds. Temperature: Can disrupt weak interactions. • Example: Function of an enzyme depends on pH, temperature, and salt concentration. Nucleic acids store and transmit hereditary information for all living things There are two types of nucleic acids in living things: A. Deoxyribonucleic Acid (DNA) Contains genetic information of all living organisms. Has segments called genes which provide information to make each and every protein in a cell Double-stranded molecule which replicates each time a cell divides. B. Ribonucleic Acid (RNA) Three main types called mRNA, tRNA, rRNA RNA molecules are copied from DNA and used to make gene products (proteins). Usually exists in single-stranded form. DNA and RNA are polymers of nucleotides that determine the primary structure of proteins Nucleotide: Subunits of DNA or RNA. Nucleotides have three components: 1. Pentose sugar (ribose or deoxyribose) 2. Phosphate group to link nucleotides (-PO4) 3. Nitrogenous base (A,G,C,T or U) Purines: Have 2 rings. Adenine (A) and guanine (G) Pyrimidines: Have one ring. Cytosine (C), thymine (T) in DNA or uracil (U) in RNA. James Watson and Francis Crick Determined the 3D Shape of DNA in 1953 Double helix: The DNA molecule is a double helix. Antiparallel: The two DNA strands run in opposite directions. Strand 1: 5’ to 3’ direction (------------>) Strand 2: 3’ to 5’ direction (<------------) Complementary Base Pairing: A & T (U) and G & C. A on one strand hydrogen bonds to T (or U in RNA). G on one strand hydrogen bonds to C. Replication: The double-stranded DNA molecule can easily replicate based on A=T and G=C --- pairing. SEQUENCE of nucleotides in a DNA molecule dictate the amino acid SEQUENCE of polypeptides DNA: Double Helix of Two Complementary Strands Held Together by H-Bonds A Gene is a specific segment of a DNA molecule with information for cell to make one polypeptide DNA (transcribed into single stranded RNA “copy”) ! ! mRNA (single stranded “copy” of the gene) ! ! Polypeptide (mRNA message translated into polypeptide) Genetic Information Flow: DNA to RNA to Protein Lipids: Fats, phospholipids, and steroids Diverse groups of compounds. Composition of Lipids: C, H, and small amounts of O. Functions of Lipids: Biological fuels Energy storage Insulation Structural components of cell membranes Hormones Lipids: Fats, phospholipids, and steroids 1. Simple Lipids: Contain C, H, and O only. A. Fats (Triglycerides). Glycerol : Three carbon molecule with three hydroxyls. Fatty Acids: Carboxyl group and long hydrocarbon chains. Characteristics of fats: Most abundant lipids in living organisms. Hydrophobic (insoluble in water) because nonpolar. Economical form of energy storage (provide 2X the energy/weight than carbohydrates). Greasy or oily appearance. Fats (Triglycerides): Glycerol + 3 Fatty Acids Lipids: Fats, phospholipids, and steroids Types of Fats Saturated fats: Hydrocarbons saturated with H. Lack -C=C- double bonds. Solid at room temp (butter, animal fat, lard) Unsaturated fats: Contain -C=C- double bonds. Usually liquid at room temp (corn, peanut, olive oils) Saturated Fats Contain Saturated Fatty Acids 2. Complex Lipids: In addition to C, H, and O, also contain other elements, such as phosphorus, nitrogen, and sulfur. A. Phospholipids: Are composed of: Glycerol 2 fatty acid Phosphate group Amphipathic Molecule Hydrophobic fatty acid “tails”. Hydrophilic phosphate “head”. Function: Primary component of the plasma membrane of cells Phospholipids: Amphipathic Molecules In Water Phospholipids Spontaneously Assemble into Organized Structures B. Steroids: Lipids with four fused carbon rings Includes cholesterol, bile salts, reproductive, and adrenal hormones. Cholesterol: The basic steroid found in animals • Common component of animal cell membranes. • Precursor to make sex hormones (estrogen, testosterone) • Generally only soluble in other fats (not in water) • Too much increases chance of atherosclerosis. C. Waxes: One fatty acid linked to an alcohol. Very hydrophobic. Found in cell walls of certain bacteria, plant and insect coats. Help prevent water loss. Cholesterol: The Basic Steroid in Animals