Empirical Formulas Worksheet
advertisement

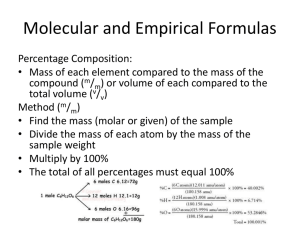
Chemistry Mr. Tothero Name: _________________________________ Date: ______ Chapter 7 Part 2 Worksheet Packet Formula Mass and Percentage Composition Worksheet FORMULA MASS Calculate the formula mass of each substance in a.m.u. and to 2 decimal places. Show your work! 1. KMnO4 4. iron(III) sulfide 2. triboron heptachloride 5. Al(NO3)3 3. 6. carbonic acid H3PO4 PERCENTAGE COMPOSITION Calculate the percentage of each element in the given compound as indicated by each problem. Show your work! Use a separate piece of paper!! 7. Calculate the percentage of copper in each compound below. a) CuO b) copper (II) phosphate 8. Calculate the percentage of carbon in each compound below. a) propane b) Al(C2H3O2)3 9. Calculate the percentage of water in each hydrated compound below. a) Na2CO3 • 10 H2O b) zinc sulfate heptahydrate Moles Calculations Review Problems Worksheet 6.02 × 1023 𝑝𝑎𝑟𝑡𝑖𝑐𝑙𝑒𝑠 = 1𝑚𝑜𝑙𝑒 = 𝑚𝑜𝑙𝑎𝑟 𝑚𝑎𝑠𝑠 (𝑓𝑟𝑜𝑚 𝑝𝑒𝑟𝑖𝑜𝑑𝑖𝑐 𝑡𝑎𝑏𝑙𝑒) Instructions: Perform each calculation showing all work and indicating your answer in the proper units. 1. How many molecules of N2O are present in 0.765 moles? 2. How many moles of FeCl3 are equal to 3.12 x 1024 formula units? 3. How many grams of C5H12 are equal to 4.215 moles? 4. How many moles of Na2SO4 are equal to 25.00 g Na2SO4? 5. How many formula units of Cu(CN)2 are present in 15.00 g Cu(CN)2? 6. How many grams of P3O5 contain 5.50 x 1022 molecules? 7. How many formula units of Na3N are equal to 3.86 moles? 8. How many grams of SO3 are equal to 0.075 moles? 9. How many moles of Mg3(PO4)2 are equal to 243.66 g of Mg3(PO4)2? 10. How many grams of C8H18 contain 4.77 x 1023 molecules? 11. How many moles of H3PO3 are equal to 55.80 g H3PO3? 12. How many molecules of H2CO3 are present in 38.54 g H2CO3? Empirical Formulas Worksheet I. Write the empirical formula for each chemical formula shown below. a) B4H10 ___________ d) C6H3Cl3 _____________ b) C12H8O4 __________ e) C8H16 _____________ c) NH3 ___________ f) H2SO4 _____________ II. Determine the empirical formulas for the compounds with the following analyses. Show your work. a) 65.95% Barium, 34.05% Chlorine b) 36.48% Sodium, 25.44% Sulfur, 38.08% Oxygen c) 93.56% Niobium, 6.44% Oxygen d) 41.39% Carbon, 3.47% Hydrogen, 55.14% Oxygen e) 26.52% Chromium, 24.53% Sulfur, 48.95% Oxygen Molecular Formulas Worksheet Show your work for each problem! 1) The empirical formula of a compound is CH2O. If the formula mass of the compound is 150.20 amu, what is the molecular formula? 2) The empirical formula of a compound is C3H2O. If the formula mass of the compound is 162.15 amu, what is the molecular formula? 3) The empirical formula of a compound is CHO. If the formula mass of the compound is 116.08 amu, what is the molecular formula? 4) The empirical formula of a compound is Cr2(SO4)3. If the formula mass of the compound is 392.21 amu, what is the molecular formula? 5) The analysis of a compound indicates that it is composed of 82.22 % Nitrogen and 17.78% Hydrogen. The formula mass of the compound is 34.08 amu. What are the empirical and molecular formulas of the compound?