Chapter 3: Water and the Fitness of the Environment
advertisement

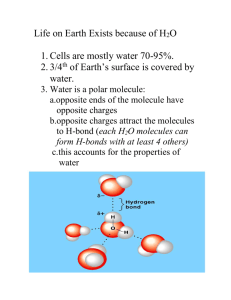
Dangerous Chemicals http://www.dhmo.org/facts.html Chapter 3 Water & the Fitness of the Environment Water supports all life! Properties of Water ¾ of the Earth is covered in water Composition of cells = 70-95% water 3 billion years before life on land All life on Earth requires water Brainstorming What are the properties of water that make is so suitable for supporting life? Polarity of water molecules results in hydrogen bonding Water molecule=polar molecule Water molecules form hydrogen bonds These H-bonds are what gives water its unique properties LE 3-2 What are the 4 emergent properties of water that contribute to Earth’s fitness for life? I. Cohesion; adhesion; surface tension II. High specific heat III. Expansion upon freezing IV. Versatility as a solvent Cohesion Cohesion: Water molecules stick together Adhesion: Water molecules stick to other substances Water-conducting cells LE 3-3 100 µm Surface tensionhow hard it is to break the surface of a liquid Surface tension is related to cohesion Moderation of Temperature H2O absorbs heat from warmer air H2O releases heat from cooler air Water absorbs or releases a large amount of heat with only a slight change in its own temperature Heat and Temperature Heat- measure of the total amount of kinetic energy due to molecular motion Temperature measures intensity of heat due to the average kinetic energy of molecules Which has more heat? An ocean (30°C) vs Blue whale (38°C) A boiling kettle of water (100°C) vs an Iceberg (-20°C) Water’s High Specific Heat The specific heat of a substance is the amount of heat that must be absorbed or lost for 1 g of that substance to change its temperature by 1ºC Specific heat of water – 1 cal/g/°C Water has an unusually high specific heat ◦ Heat is absorbed when H-bonds break ◦ Heat is released when H-bonds form Brainstorm Take a few minutes to write a list of reasons for why the specific heat of water is important for biological processes. Be ready to explain your answer Evaporative Cooling Evaporation- liquid to gas Heat of vaporization- heat a liquid must absorb for 1 g to be converted to gas Liquid evaporates Remaining liquid becomes cooler Evaporative cooling… ◦ Stabilizes temperature of bodies of water ◦ Helps organisms maintain body heat Insulation of Bodies of Water by Floating Ice Frozen water is less dense than liquid water (it floats) ◦ As the temperature of water decreases, hbonds are not broken as fast More hydrogen bonds greater spacing The density of ice < liquid water What would happen if ice sank? Why is Water the Solvent of Life? Solution-liquid homogeneous mix of substances Solvent- dissolving agent of a solution Solute- substance dissolved Water is a versatile solvent due to its polarity An aqueous solution is one in which water is the solvent Water forms hydrogen bonds with ionic solutes Hydration shell Water can also dissolve: Polar molecules Some proteins (b) – (c) Lysozyme molecule in an aqueous environment. (a) Lysozyme molecule in a nonaqueous environment . What substances will NOT dissolve in water? Hydrophobic Substances Hydrophobic substances are nonpolar They do not dissolve in water (which is polar) Hydrophilic substances are polar They easily dissolve in water “Like dissolves like” Solute Concentration in Aqueous Solutions Chemical reactions depend on collisions of molecules and therefore on the concentration of solutes in an aqueous solution. What is a mole? Concentration Molarity (M)- # of moles of solute per L of solution Molecular mass - sum of all masses of all atoms in a molecule Avogadro’s Number – the number of atoms/molecules per mole. ◦ 6.02 x 10^23 How would you make 1 liter of a 0.5 M solution of NaCl? How many moles of NaCl do you need? What is the molecular mass of NaCl? ◦ Na - ~23 g/mol ◦ Cl - ~35.5 g/mol How many grams of NaCl do you need? Extra Practice How many molecules are in 60 grams of NaCl? Determine the molarity of the solution: 100 g KCl dissolved into 0.75 L of solution. How many grams of KCl are necessary to get 0.5 L of a 0.10M solution? Water acts as both an acid and a base. Water dissociates to form H+ (or H3O+) and OH- ions. At equilibrium – 10^-7M Hydronium (H3O+) and hydroxide (OH-) ions are extremely reactive. Water is both an acid and a base. Bronsted Acid – A substance that donates a proton in an aqueous solution. Bronsted Base – Proton acceptor. Amphoteric – A substance that can act as either an acid or a base. Amphiprotic – A substance that can either donate or accept a proton. Concentrations of acids and bases. Concentrations of H+ and OH- are equal in pure water Product is constant: [H+][OH-] = 10^-14 Dissolving acids in aqueous solutions increases the concentration of H+ ions. Bases [OH-] > [H+] ◦ NaHO Na+ and OH◦ NH3 NH4+ and OH- Practice What is [H+] if [OH-] = 10^-8M? ◦ [H+][OH-] = 10^-14 ◦ 10^-14/[OH-] = [H+] ◦ 10^-14/10^-8 = [H+] = 10^-6M pH is a measure of acidity pH + pOH = 14 pH = -log [H+] ◦ Acid- pH < 7 ◦ Base- pH > 7 pOH = -log [OH-] ◦ Acid – pOH > 7 ◦ Base – pOH < 7 pH practice What is the pH of a 0.10M solution of NaOH? ◦ NaOH Na+ + OH-; determine the ratio of moles. ◦ pOH = -log [OH-] ◦ pH = 14 - pOH pH practice What is the concentration of a solution of HCl if the pH = 3? What makes an acid strong? Strong acids/bases dissociate completely Weak acids/bases dissociate until equilibrium is reached Examples: ◦ Strong base – NaOH ◦ Weak base – NH3 pH Scale 0 Increasingly Acidic [H+] > [OH–] 1 Neutral [H+] = [OH–] Battery acid 2 Digestive (stomach) juice, lemon juice 3 Vinegar, beer, wine, cola 4 Tomato juice 5 Black coffee Rainwater 6 Urine 7 Pure water Human blood 8 Seawater Increasingly Basic [H+] < [OH–] 9 10 Milk of magnesia 11 Household ammonia 12 Household bleach 13 Oven cleaner 14 LE 3-8 Effect of pH on living cells Most biological liquids – 6 ≤ pH ≤ 8 Internal pH of most cells ~7 Brainstorming: What would happen to a cell if the internal pH was decreased by 1? Why? Buffers The internal pH of most living cells must remain close to pH 7 Buffers help to minimize changes in concentrations of H+ & OH- in a solution Buffer: a solution composed of a weak acid and its conjugate base (or a weak base and its conjugate acid) The Threat of Acid Precipitation Acid precipitation (ppt)- rain, snow, or fog w/ a pH < 5.6; caused by mixing pollutants w/ water in the air Acid precipitation can damage life in lakes and streams Effects of acid precipitation on soil chemistry are contributing to the decline of some forests How do buffers work? NH3(g)+H2O(l)→NH4+(aq)+OH−(aq) NH3 = weak base NH4+ = conjugate acid When an acid is added to a buffer solution, the buffer is ready to accept protons (H+). When a base is added, the buffer is ready to donate protons. Think about it… Why can’t buffers be made with strong acids/bases? Le Chatelier’s Priciple For a system that is at equilibrium, changes in (concentration, temperature, pressure, volume) will cause the reaction to shift either forward or toward the reverse reaction in order to counteract the change. 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 More acidic Acid rain Normal rain More basic LE 3-9 Exam practice Buffers are substances that help resist shifts in pH by A) releasing H+ in acidic solutions. B) donating H+ to a solution when they have been depleted. C) releasing OH- in basic solutions. D) accepting H+ when the are in excess. E) both B and D Exam practice Buffers are substances that minimize changes in the concentrations of H+ and OH- ions in a solution The answer is E Exam practice Which of the following is responsible for the cohesive property of water? ◦ A. H-bonds b/w the oxygen atoms of 2 adjacent water molecules ◦ B. covalent bonds b/w Hydrogen atoms of 2 adjacent water molecules ◦ C. H-bonds b/w the oxygen atom of 1 water molecule and the hydrogen atom of another ◦ D. covalent bonds b/w O atom of 1 water molecule & H atom of another ◦ E. H-bonds b/w water molecules and other types of molecules Exam practice The cohesive property of water is due to the hydrogen bonding between water molecules, specifically the hydrogen bonds b/w the oxygen atom of one water molecule and the hydrogen atom of another water molecule The answer is C Your pH investigation lab outcome should look like this