C - Solon City Schools
advertisement

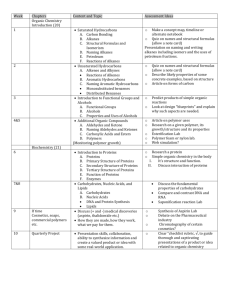
Honors Chemistry Unit 7 Organic Chemistry Allotropes Isomers Hydrocarbons o Alkanes o Alkenes o Alkynes o Aromatics Alkyl Halides Ethers Alcohols Aldehydes Ketones Carboxylic Acids Esters Amines Parent Chain: Methane CH4 Ethane C2H6 Propane C3H8 Butane C4H10 Pentane C5H12 Hexane C6H14 Heptane C7H16 Octane C8H18 Nonane C9H20 Decane C10H22 Undecane C11H24 Dodecane C12H26 Branches (one less Hydrogen than Parent Chain): all prefixes can be made into branches Methyl CH3 Ethyl C2H5 Introduction of parent chain and branches Organic Chemistry Hydrocarbons contain Carbon and Hydrogen only If all bonds on the carbons are single bondsdrocarbon Alkanes Have form CnH2n+2 (where n = # of carbons) Ending of the name is “ane” Name Molecular Formula CnH2n+2 Methane Ethane Propane Butane 2-Methylpropane Structural Formula Model Example 1: 3-ethylheptane Example 2: 2,7-dimethylnonane Example 3: 4-ethyl-2,4,5-trimethyloctane Example 4: 3,3,4,4-tetraethyl-2,2,5,5-tetramethylhexane Cycloalkanes All single bonds Have form CnH2n Ends of chain bond together (lose 2 hydrogens) Examples: More practice Naming Alkanes Drawing Alkanes Unsaturated Hydrocarbons = not all carbons have 4 single bonds (double or triple bonds) Alkenes Structure contains double bonds Have form CnH2n Name Molecular Formula CnH2n Ethene Propene 2-Butene Structural Formula Model Drawing and naming Alkene structures: Alkynes Structure contains triple bonds Have form CnH2n-2 Name Molecular Formula CnH2n-2 Ethyne 1-Propyne 1-Butyne Naming and drawing Alkynes: Structural Formula Aromatic Hydrocarbons Mu st contain at least one benzene ring Naming Aromatics Date__________ Name ____________________________________ Modern Chemistry Lab Practical Unit on "Organic Chemistry" I. Problem: What are some of the differences between various organic compounds? You will be given some notes and then asked to design a model of these organic compounds including: alkanes, isomers of alkanes, alkenes, alkynes, alcohols, organic acids, aldehydes, ketones and some nitrogen containing organic compounds. II. Background Information: Alkanes are saturated hydrocarbons with a formula of CnH2n+2. Isomers of alkanes would have the same formula but different structures. Other information will be given at the beginning of each day of this lab practical. III. Hypothesis: IV. Test The Hypothesis: Materials: make certain your kit contains the following in the right location. If it does not, the previous user will be marked at least 1 point off their next assignment grade each day there is a problem. Each day you work with the kit, you are to sign the paper in the kit with your class numbers and date. 10 black spheres 2 blue spheres 28 yellow spheres 6 red spheres 4 green spheres 2 orange spheres 2 white spheres2 purple spheres- carbon \ place these on the small side of your kit nitrogen / hydrogen \ oxygen \ chlorine \ place these on the larger side bromine / of your kit fluorine / (all colors are to be placed together) iodine / 30 small wooden pegs - H bonds only \ 10 large wooden pegs - other single bonds \ place these in the 9 metal springs double or triple bonds / center of your kit or stress bonds / V. Observations: The first student lab team that has the structure correct will be initialed by the instructor and receive a grade of 2 points out of 1 point. These students will then have their names placed on the board and will be teaching assistants for that organic structure. They will be responsible to grade and initial the structures of other lab teams. Each structure that is designed and named by you is worth 1 point. The following is the grading criteria: 2 points = graded by the instructor and perfect, this team assists in grading 1 point = other perfect scores 0 points = 1 or more mistakes in the structure and/or name Assignment # ____ Name ____________________________________ Class Number ______ Date ________ Name ____________________________________ Class Number ______ Kit # _______ Honors Chemistry Lab Practical "Organic Chemistry" Alkanes – Straight and Branched (If absent - write an I-search paper describing the major uses of each of the first 10 alkanes. This must be written in the first person. Answer the 3 questions on page 7 using the criteria of an I-Search.) (2 points, ½ point each) Grade/Initials _______________ 1. hexane _______________ 2. 3-methylpentane _______________ 3. 2, 4-dimethylhexane _______________ 4. 2, 2-dimethylpentane _______________ Bonus ½ Point: 3-ethyl-2, 4-dimethylhexane (only for 1st three teams who are correct, one attempt per team) _______________ 1 point lost for problems with your kit /2 _______________Total points for Straight and Branched Alkanes Information on Doing an “I-Search” Paper to Make-Up a Missed Organic Chem Modeling Lab: 1. An "I-Search” paper is telling the story of what you did in your search, those happenings and facts crucial to your hunt for information on your assigned topic. Normally, you are to write 150 words (one page) for each section of the lab you missed. 2. The paper is to be typed and divided into three parts (each section is to be titled as below): a. What I knew when I started my search. b. The facts I found in my search and what I learned. c. What I found the most interesting with what I learned. 3. If your make-up paper does not answer these questions, the best score you can receive is half credit. Name ______________________________________________ Alkane Worksheet Based on the given IUPAC name, give the structural formula for each of the following molecules (number the carbons): Name 1. dodecane 2. 2-methylheptane 3. 2,3,4-trimethylhexane 4. 3-methylpentane 5. 3-ethyl,2,4,4-trimethylnonane 6. 3-butyl,2,4-diethyl,1-methyl cyclobutane Structural Formula Give the IUPAC name for each of the following molecules (number the carbons): Name Structural Formula 1. CH3-CH2-CH2-CH2-CH2-CH2-CH3 2. CH3 CH3-CH-CH2-CH3 3. CH3 CH3 CH3 CH3-CH-CH2-CH-CH-CH3 4. CH2-CH3 CH3 5. 6. CH3 CH3 CH2 CH2 CH3-CH2-CH-CH2-CH2-CH-CH2-CH3 CH3 CH3 CH3 CH2 CH2 CH2 CH3-CH-CH-CH2-CH2-CH-CH2-CH3 Introduce Isomers Assignment # ____ Name ____________________________________ Class Number ________ Date ________ Name ____________________________________ Class Number ________ Kit # _______ Honors Chemistry Lab Practical "Organic Chemistry" Isomerism (If absent - write an I-search paper on organic isomers. This must be written in the first person and be 100 words. Answer the 3 questions on page 7 of your grade sheets concerning an I-Search.) (2 points, ½ point each) Grade/Initials: Do not use –iso or –neo. Use the naming system found in 19.4. _______________ 1. butane isomer #1 (simple) (0.25 point) name _____________________________________ (0.25 point) _______________ 2. butane isomer #2 (one branch) (0.25 point) name _____________________________________ (0.25 point) _______________ 3. pentane isomer #2 (one branch) (0.25 point) name _____________________________________ (0.25 point) _______________ 4. pentane isomer #3 (two branches) (0.25 point) name _____________________________________ (0.25 point) _______________ Bonus ½ Point: any isomer of hexane Name ___________________________________ not done by another team put the name on the board) (0.25 point) (Teacher checks) (0.25 point) _______________ 1 point lost for problems with your kit / 2 _______________ Total points for Isomerism Name _____________________________________________ Draw and name the 18 isomers of Octane Octane: 1. Methyl heptane: 1. 2. 3. Dimethyl hexane: 1. 2. 3. 4. 5. 6. Ethyl hexane: 1. Trimethyl pentane: 1. 2. 3. 4. Methyl ethyl pentane: 1. 2. Tetramethyl butane: 1. Assignment # ____ Name ____________________________________ Class Number ________ Date ________ Name ____________________________________ Class Number ________ Kit # _______ Honors Chemistry Lab Practical "Organic Chemistry" Unsaturated Hydrocarbons (If absent - write an I-search paper unsaturated hydrocarbons. This must be written in the first person and be 100 words. Answer the 3 questions on page of your packet concerning an I-Search.) (3 points, ½ point each) Grade/Initials _______________ 1. 1-butene _______________ 2. 2-butyne _______________ 3. 2-methylpropene _______________ 4. 3-methylbutene _______________ 5. 1, 3-pentadiene _______________ 6. 4-methylpentyne _______________ Bonus ½ Point: chloroethene (the monomer of polyvinyl chloride) ((1st three groups correct get bonus point) ( one attempt per team) (Teacher checks) _______________ 1 point lost for problems / 3 _____________ Total points for Unsaturated Hydrocarbons Name ______________________________________________ Alkene Alkyne Assignment Based on the given IUPAC name, give the structural formula for each of the following molecules (number the carbons): Name 1. 3-heptene 2. 3-methyl-butyne 3. 4,5,5-trimethyl-1-hexene 4. 3-ethyl-2,4-dimethyl-2-pentene 5. 5-methyl-1,3-hexadiyne 6. 1,2,4-trimethyl-1-cyclopentene 7. 2,4,5-trimethyl-1,3-cyclohexadiene Structural Formula Give the IUPAC name for each of the following molecules (number the carbons): Name Structural Formula 1. C–C–C=C-C-C 2. C C=C–C–C-C 3. C C C-C-C - C-C=C 4. 5. 6. C C–C-C C C C C C -C = C–C=C-C–C C C C C C CΞC-C - C-C -C≡C 7. C C -C Assignment # ____ Name ____________________________________ Class Number ________ Date ________ Name ____________________________________ Class Number ________ Kit # _______ Honors Chemistry Lab Practical "Organic Chemistry" Mixed Aromatic Hydrocarbons, Alkyl Halides and Hydrocarbons (If absent - write an I-search paper unsaturated hydrocarbons. This must be written in the first person and be 150 words. Answer the 3 questions on page of your packet concerning an I-Search.) (3 points, ½ point each) Grade/Initials _______________ 1. 2-chloropropane _______________ 2. 2-bromo-3,4 -dichloropentene _______________ 3. 5-bromo-1- chloro-4,4-diiodo-5-methyl-2- hexyne _______________ 4. ethylbenzene _______________ 5. 1-ethyl-3-methylbenzene _______________ 6. 1,2,4-trimethylbenzene _______________ 1 point lost for problems / 3 _____________ Total points for Aromatics and Alkyl Halides Alkane, Alkene, Alkyne, Cyclos and Aromatic Review Sheet Section 1: Draw the following structures: 1. 1-ethyl-2-methyl-cyclohexane 2. 2,3-dimethyl-1,5-heptadiene 3. 3-bromo-4,5-dichloro-6-iodo-undecane 4. 1-butyl-3-propyl-benzene 5. 3.3.4-trifluoro-1,6-octadiyne 6. 1-ethyl-4-methyl-1,3-cycloctadiene 7. 1-bromo-5,6-dichloro-3-fluoro-2,4-dimethyl-benzene Section 2: Name the following structures Petroleum Oil and natural gas touch our lives in countless ways every day. Together, they supply 65 percent of our nation’s energy. They fuel our cars, heat our homes and cook our food. But did you know that oil and natural gas also help generate the electricity that powers our daily lives? Or that crude oil supplies the building blocks for everything from dent-resistant car fenders to soft drink bottles to camping equipment? “Hy”-CARB Crude oils are mixtures of many different substances, often difficult to separate, from which various petroleum products are derived, such as: gasoline, kerosene propane, fuel oil, lubricating oil, wax, and asphalt. These substances are called hydrocarbons. Hydrocarbons may be gaseous, liquid, or solid at normal temperature and pressure, depending on the number and arrangement of the carbon atoms in their molecules. Those with up to 4 carbon atoms are gaseous; those with 20 or more are solid; those in between are liquid. Crude oils are liquid but may contain gaseous or solid compounds (or both) in solution. The heavier a crude oil (i.e. the more carbon atoms its molecules contain) the closer it is to being a solid and this may be especially noticeable as its temperature cools. Light oils will remain liquid even at very low temperatures. Crude “Development” Oil is formed from the remains of tiny plants and animals (plankton) that died in ancient seas between 10 million and 600 million years ago. After the organisms died, they sank into the sand and mud at the bottom of the sea. Over the years, the organisms decayed in the sedimentary layers. In these layers, there was little or no oxygen present. So microorganisms broke the remains into carbon-rich compounds that formed organic layers. The organic material mixed with the sediments, forming fine-grained shale, or source rock. As new sedimentary layers were deposited, they exerted intense pressure and heat on the source rock. The heat and pressure distilled the organic material into crude oil and natural gas. The oil flowed from the source rock and accumulated in thicker, more porous limestone or sandstone, called reservoir rock. Movements in the Earth trapped the oil and natural gas in the reservoir rocks between layers of impermeable rock, or cap rock, such as granite or marble. Black gold, Texas tea! Crude oil is the term for "unprocessed" oil, the stuff that comes out of the ground. It is also known as petroleum. Crude oils vary in color, from clear to tar-black, and in viscosity, from watery to almost solid. Crude oils are such a useful starting point for so many different substances because they contain hydrocarbons. Hydrocarbons are molecules that contain hydrogen and carbon and come in various lengths and structures, from straight chains to branching chains to rings. There are two things that make hydrocarbons exciting to chemists: Hydrocarbons contain a lot of energy. Many of the things derived from crude oil like gasoline, diesel fuel, paraffin wax and so on take advantage of this energy. o Hydrocarbons can take on many different forms. The smallest hydrocarbon is methane (CH4), which is a gas that is a lighter than air. Longer chains with 5 or more carbons are liquids. Very long chains are solids like wax or tar. By chemically cross-linking hydrocarbon chains you can get everything from synthetic rubber to nylon to the plastic in tupperware. Hydrocarbon chains are very versatile! The major classes of hydrocarbons in crude oils include: o Alkanes: methane, ethane, propane, butane, pentane, hexane Aromatics: benzene, napthalene Cycloalkanes: cyclohexane, methyl cyclopentane Alkenes: ethylene, butene, isobutene Divide and Conquer The problem with crude oil is that it contains hundreds of different types of hydrocarbons all mixed together. You have to separate the different types of hydrocarbons to have anything useful. Fortunately there is an easy way to separate things, and this is what oil refining is all about. Refining crude oil involves two kinds of processes to produce the products so essential to modern society. First, there are physical processes that simply refine the crude oil (without altering its molecular structure) into useful products such as lubricating oil or fuel oil. Second, there are chemical or other processes that alter the molecular structure and produce a wide range of products, some of them known by the general term petrochemicals. Chain Gang The oil refining process starts with a fractional distillation column. Different hydrocarbon chain lengths all have progressively higher boiling points, so they can all be separated by distillation. In an oil refinery, crude oil is heated and the different chains are pulled out according to their vaporization temperatures. Each different chain length has a different property that makes it useful in a different way. “Piece” Out To understand the diversity contained in crude oil, and to understand why refining crude oil is so important in our society, look through the following list of products that come from crude oil: Petroleum gas - used for heating, cooking, making plastics small alkanes (1 to 4 carbon atoms) o boiling range = less than 104 degrees Fahrenheit / 40 degrees Celsius o often liquefied under pressure to create LPG (liquefied petroleum gas) Naphtha or Ligroin - intermediate that will be further processed to make gasoline o o mix of 5 to 9 carbon atom alkanes o boiling range = 140 to 212 degrees Fahrenheit / 60 to 100 degrees Celsius Gasoline - motor fuel o liquid o mix of alkanes and cycloalkanes (5 to 12 carbon atoms) o boiling range = 104 to 401 degrees Fahrenheit / 40 to 205 degrees Celsius Kerosene - fuel for jet engines and tractors; starting material for making other products o liquid o mix of alkanes (10 to 18 carbons) and aromatics o boiling range = 350 to 617 degrees Fahrenheit / 175 to 325 degrees Celsius Gas oil or Diesel distillate - used for diesel fuel and heating oil o liquid o alkanes containing 12 or more carbon atoms o boiling range = 482 to 662 degrees Fahrenheit / 250 to 350 degrees Celsius Lubricating oil - used for motor oil, grease, other lubricants o o o Heavy liquid long chain (20 to 50 carbon atoms) alkanes, cycloalkanes, aromatics boiling range = 572 to 700 degrees Fahrenheit / 300 to 370 degrees Celsius gas or Fuel oil - used for industrial fuel; starting material for making other products o liquid o long chain (20 to 70 carbon atoms) alkanes, cycloalkanes, aromatics o boiling range = 700 to 1112 degrees Fahrenheit / 370 to 600 degrees Celsius Residuals - coke, asphalt, tar, waxes; starting material for making other products o o o solid multiple-ringed compounds with 70 or more carbon atoms boiling range = greater than 1112 degrees Fahrenheit / 600 degrees Celsius Break it Down! Very few of the components come out of the fractional distillation column ready for market. Many of them must be chemically processed to make other fractions. For example, only 40% of distilled crude oil is gasoline; however, gasoline is one of the major products made by oil companies. Rather than continually distilling large quantities of crude oil, oil companies chemically process some other fractions from the distillation column to make gasoline; this processing increases the yield of gasoline from each barrel of crude oil. Chemical Processing You can change one fraction into another by one of three methods: o o o Cracking - breaking large hydrocarbons into smaller pieces. Unification - combining smaller pieces to make larger ones. Alteration - rearranging various pieces to make desired hydrocarbons. Cracking up Cracking breaks large chains into smaller chains. There are several types of cracking: Thermal - you heat large hydrocarbons at high temperatures (sometimes high pressures as well) until they break apart. Coking - residual from the distillation tower is heated to temperatures above 900 degrees Fahrenheit/482 degrees Celsius until it cracks into heavy oil, gasoline and naphtha. When the process is done, a heavy, almost pure carbon residue is left (coke); the coke is cleaned from the cokers and sold. Catalytic - uses a catalyst to speed up the cracking reaction. Catalysts include zeolite, aluminum hydrosilicate, bauxite and silica-alumina. After various hydrocarbons are cracked into smaller hydrocarbons, the products go through another fractional distillation column to separate them. Come together Sometimes you need to combine smaller hydrocarbons to make larger ones -- this process is called unification. The major unification process is called catalytic reforming and uses a catalyst (platinum) to combine low weight naphtha into aromatics, which are used in making chemicals and in blending gasoline. Sometimes, the structures of molecules in one fraction are rearranged to produce another. Commonly, this is done using a process called alkylation. In alkylation, low molecular weight compounds, such as propylene and butylene, are mixed in the presence of a catalyst such as hydrofluoric acid or sulfuric acid. The products of alkylation are high octane hydrocarbons, which are used in gasoline blends. Take it to the Cleaners Distillated and chemically processed fractions are then treated to remove impurities, such as organic compounds containing sulfur, nitrogen, oxygen, water, dissolved metals and inorganic salts. Treating is usually done by passing the fractions through the following: A column of sulfuric acid - removes unsaturated hydrocarbons (those with carbon-carbon double-bonds), nitrogen compounds, oxygen compounds and residual solids (tars, asphalt) o An absorption column filled with drying agents to remove water o Sulfur treatment and hydrogen-sulfide scrubbers to remove sulfur and sulfur compounds After the fractions have been treated, they are cooled and then blended together to make various o products, such as: o o o o o o o Gasoline of various grades, with or without additives Lubricating oils of various weights and grades (e.g. 10W-40, 5W-30) Kerosene Jet fuel Diesel fuel Heating oil Chemicals of various grades for making plastics and other polymers Burn Baby Burn! Alkanes are less reactive than other hydrocarbons because of the stability of their single covalent bonds. One reaction alkanes do undergo is combustion. Because alkanes make up a large portion of gaseous and liquid fossil fuels, combustion is their most important reaction. Complete combustion of hydrocarbons produces energy, CO 2, and H2O. The reaction for combustion of methane produces 890kJ/mol of energy. CH4 + 2 O2 CO2 + 2 H2O One concern about the combustion of fossil fuels is their possible contribution to the greenhouse effect. CO2 is one of the atmospheric molecules that absorbs infrared radiation. Increased levels of CO2 through the combustion of fossil fuels may increase the amount of infrared energy absorbed by the atmosphere to a level that can increase the average temperature of Earth. Engines can be powered by gasoline combustion. When fuel ignites spontaneously before it is reached by the flame front, there is a decrease in the amount of power gained, and engine knocking results. Straight-chain hydrocarbons are more likely to ignite spontaneously than branched-chain hydrocarbons. This tendency is the basis for the octane rating scale. The octane rating of a fuel is a measure of its burning efficiency and its antiknock properties. The octane rating scale is based on mixtures of 2,2,4-trimethylpentane, a highly branched alkane, and heptane, a straightchain alkane. The term octane comes from the common name of 2,2,4trimethylpentane, isooctane. Pure 2,2,4-trimethylpentane is very resistant to knocking and is assigned an octane number of 100. Pure heptane has an octane number of 0 and burns with a lot of knocking. Increasing the percentage of branched-chain alkanes in gasoline is one way to increase octane rating. The octane rating on gasoline pumps is shown in the figure to the right Name ____________________ “Fuel” for Thought! 1. Indentify/sort the hydrocarbons that are gas, liquid, or solid at room temperature. Gas Solid Liquid 2. Describe the conditions necessary to form crude oil. 3. Identify the components of gasoline (or petrol as the Australians say). 4. Describe different methods used to increase the amount of gasoline obtained from crude oil. 5. Describe how various impurities are removed from the fractions of crude oil. 6. Identify and describe how you use 5 products made from crude oil. 7. What does the octane rating mean? 8. What does a high octane number indicate about the composition (the hydrocarbons)? Other Organic Compounds Functional Group – Same Functional Group o o - Alcohols: Organic compound that General Formula Naming: o If no number is present in front of the name – presume the –OH is on carbon number 1. o If 1 -OH group - Examples: o If two or more -OH groups 2= 3= 4= Keep the ane; add the appropriate ending listed above: Examples: Classification of Alcohols: For classification – If that carbon is attached to one other carbon, it is called a ______________ C - C - C - OH If that carbon is attached to two other carbons, the compound is called a ________________________ OH C-C-C-C If that carbon is attached to three carbons, it is called a ____________________ OH C-C-C–C C Alkyl Halides: Organic compound with General Formula o o o o – – – – Naming: o Examples: Name as if the halogen is a branch Ethers: Organic compounds General Formula: - R and R’ Naming: o– o– o– Examples: