Classifying Hydrocarbons
advertisement
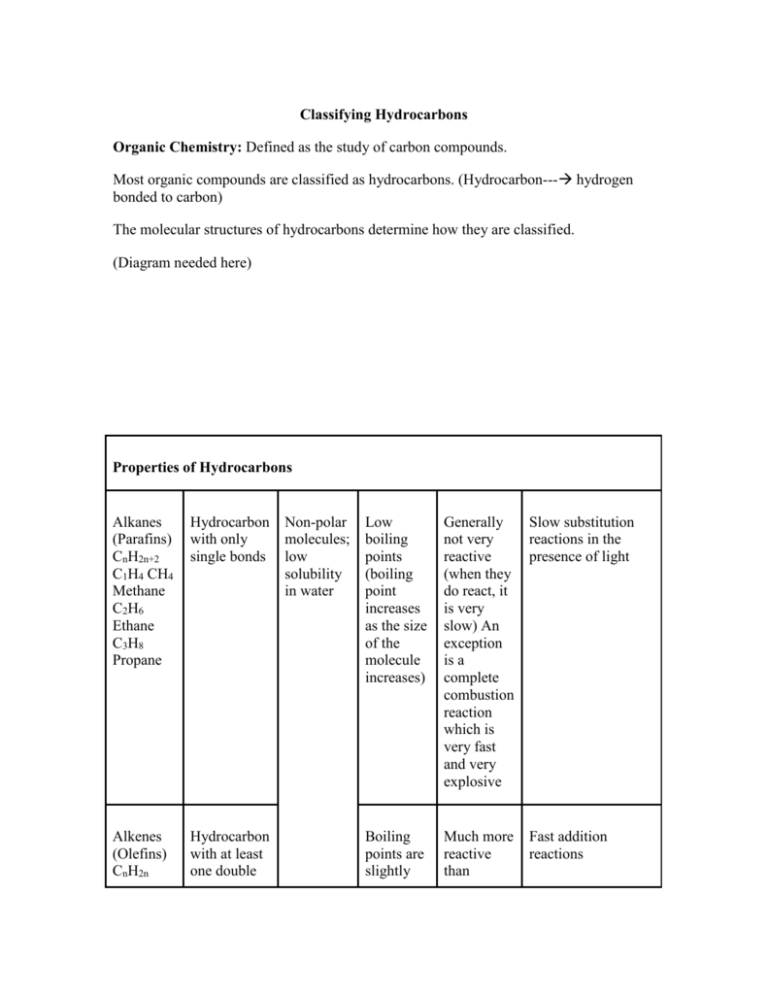
Classifying Hydrocarbons Organic Chemistry: Defined as the study of carbon compounds. Most organic compounds are classified as hydrocarbons. (Hydrocarbon--- hydrogen bonded to carbon) The molecular structures of hydrocarbons determine how they are classified. (Diagram needed here) Properties of Hydrocarbons Alkanes (Parafins) CnH2n+2 C1H4 CH4 Methane C2H6 Ethane C3H8 Propane Hydrocarbon with only single bonds Alkenes (Olefins) CnH2n Hydrocarbon with at least one double Non-polar molecules; low solubility in water Low boiling points (boiling point increases as the size of the molecule increases) Generally not very reactive (when they do react, it is very slow) An exception is a complete combustion reaction which is very fast and very explosive Slow substitution reactions in the presence of light Boiling points are slightly Much more reactive than Fast addition reactions Propene C3H6 bond; unsaturated Alkynes CnH2n-2 C3H4 Propyne Hydrocarbon with at least one triple bond less than those of alkanes alkanes Slow substitution reaction: light CH3-CH3 + Br-Br ----------- CH3-CH2Br + HBR Colourless orange (slow) colourless turns litmus paper red Fast Addition reaction: CH2-CH2 + Br-Br ---------------- CH3-CH2Br Colourless orange fast colourless Hydrocarbons Organic Chemistry: Defined as the study of carbon compounds. Most organic compounds are classified as hydrocarbons (hydrocarbon ---- hydrogen bonded to carbon). Hydrocarbon: An organ (living compound that contain on carbon and hydrogen atoms in their structure. The molecule structures of hydrocarbons determine how they are classified. Classifications: A. Aliphatic Compounds: Organic compounds with a molecular structure in which the carbon chains are straight or ringed (cyclic). They include: 1. Alkanes 2. Alkenes 3. Alkynes B. Aromatic Compounds: Organic Compounds that contain a benzene ring (C6H6(1)) Organic Nomenclature The system of nomenclature developed for organic compounds is called the IUPAC system. Hydrocarbons are studied and named based on: Bond (single/double/triple) Atom arrangement Number of atoms In this course, we will look at 4 classes of hydrocarbons; 1. Alkanes (straight chained, single bonds) 2. Alkenes (straight chained, double bonds) 3. Akynes (straight chained, triple bonds) 4. Cyclo”_____” (ring/cyclic structure) Alkanes Alkanes (Parafins) --- CnH2n+n Alkanes ------ aliphatic hydrocarbons that are composed of only hydrogen and carbon atoms, held together by single bonds. HHH H-------C-------C-------C-------H CH3---CH2----CH3 HHH Alkanes are named according to the number of carbons with the compound. All alkane names end in “ane”. # of Carbons Formula Name 1 CH4 Methane 2 C2H6(CH3CH3) Ethane 3 C3H8(CH3CH2CH3) Propane 4 C4H10(CH3(CH2)2CH3) Butane 5 C5H12(CH3(CH2)3CH3) Pentane 6 C6H14(CH3(CH2)4CH3) Hexane 7 C7H16(CH3(CH2)5CH3) Heptane 8 C8H18(CH3(CH2)6CH3) Octane 9 C9H20(CH3)(CH2)7CH3 Nonane 10 C10H22(CH3)CH2)8CH3) Decane Lewis Diagram Most organic compounds have side chains or branches. These side chains are known as alkyls and the main chain is called the parent. When naming alkyl side chains, the same nomenclature seen for the alkanes is used. Only the “ane” ending become the “yl” ending. The procedure to be followed when naming branched alkanes is as follows: 1. Find the longest continuous carbon-carbon chain. This is the parent chain. (Not always straight line). 2. Number the parent chain, starting at the end closest to the 1st branch. 3. Identify the branch and its numerical position. 4. Attach the number and name of the branch in front of the name of the parent. 5. When more than one branch exists, arrange them in alphabetical order.