Section 14.3
advertisement

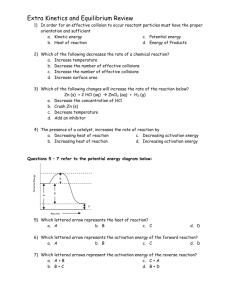
Monday April 7th: “A” Day Tuesday, April 8th: “B” Day Agenda Section 14.3: “Equilibrium Systems and Stress” Le Châtelier’s principle, common-ion effect Homework: Section 14.3 review, pg. 518: #1-7 Le Châtelier’s principle worksheet Concept Review: “Equilibrium Systems and Stress” Le Châtelier’s Principle Stress is another word for something that causes a change in a system at equilibrium. Chemical equilibrium can be disturbed by a stress, but the system soon reaches a new equilibrium. Le Châtelier’s principle: a system in equilibrium will oppose a change in a way that helps eliminate the change. Le Châtelier’s Principle Chemical equilibria respond to three kinds of stress: 1. Changes in the concentrations of reactants or products 2. Changes in temperature 3. Changes in pressure When a stress is first applied to a system, equilibrium is disturbed and the rates of the forward and backward reactions are no longer equal. Le Châtelier’s Principle The system responds to the stress by forming more products or by forming more reactants. A new chemical equilibrium is reached when enough reactants or products form. At this point, the rates of the forward and backward reactions are equal again. Increase in Reactant Concentration If you increase a reactant’s concentration, the system will respond to decrease the concentration of the reactant by changing some of it into product. Therefore, the rate of the forward reaction must be greater than the rate of the reverse reaction. The equilibrium is said to shift right, and the reactant concentration drops until the reaction reaches equilibrium. Increase in Reactant Concentration Example N2(g) + 3 H2(g) 2 NH3(g) If either N2 or H2 is increased, the equilibrium will shift to the right to try to “get rid” of the additional reactants. Decrease in Reactant Concentration If you decrease a reactant’s concentration, the system will respond to increase the concentration of the reactant by changing some of the product into reactant. Therefore, the rate of the reverse reaction must be greater than the rate of the forward reaction. The equilibrium is said to shift left, and the product concentration drops until the reaction reaches equilibrium. Decrease in Reactant Concentration Example N2(g) + 3 H2(g) 2 NH3(g) If either N2 or H2 is decreased, the equilibrium will shift to the left to try to replace the reactants that were removed. Increase in Product Concentration N2(g) + 3 H2(g) 2 NH3(g) If NH3 is increased, the equilibrium will shift to the left to try to get rid of the additional NH3 added to the system. Decrease in Product Concentration N2(g) + 3 H2(g) 2 NH3(g) If NH3 is decreased, the equilibrium will shift to the right to try to replace the product that was removed. Changes in Temperature Exothermic reactions have negative ΔH values, which means they release energy. Think of energy as a product in exothermic reactions. Endothermic reactions have positive ΔH values, which means they absorb energy. Think of energy as a reactant in endothermic reactions. Changes in Temperature Exothermic Reaction Example N2(g) + 3 H2(g) 2 NH3(g) ΔH of forward reaction = -91.8 kJ Think of it this way: N2(g) + 3 H2(g) 2 NH3(g) + energy Changes in Temperature Exothermic Reaction Example N2(g) + 3 H2(g) 2 NH3(g) + energy If the temperature is raised during an exothermic reaction, the equilibrium will shift to the left to try to relieve the added stress and additional reactants will be made. Changes in Temperature N2(g) + 3 H2(g) 2 NH3(g) + energy If the temperature is lowered during an exothermic reaction, the equilibrium will shift to the right to try to replace the energy that was removed and more products will be made. Changes in Temperature Endothermic Reaction Example N2O4 (g) 2 NO2 (g) ΔH of forward reaction= 55.3 kJ Think of it this way: N2O4 (g) + energy 2 NO2 (g) Changes in Temperature Endothermic Reaction Example N2O4 (g) + energy 2 NO2 (g) If the temperature is raised during an endothermic reaction, the equilibrium will shift to the right to relieve the added stress and more products will be made. Changes in Temperature Endothermic Reaction Example N2O4 (g) + energy 2 NO2 (g) If the temperature is lowered during an endothermic reaction, the equilibrium will shift to the left to replace the energy that was removed and more reactants will be made. Changes in Pressure Pressure has almost no effect on equilibrium reactions that are in solution. Gases in equilibrium may be affected by changes in pressure. Simply put, an increase in pressure shifts the equilibrium to favor the reaction that produces fewer gas molecules. Changes in Pressure Example 2 NOCl (g) 2 NO (g) + 1 Cl2 (g) The left side of the equilibrium contains 2 moles of gas. The right side of the equilibrium contains 3 moles of gas. An increase in pressure will shift the equilibrium to the left to produce fewer mole of gas. Changes in Pressure Example H2O (g) + CO (g) H2 (g) + CO2 (g) In this case, each side of the equilibrium contains the same number of moles of gas, 2. In such cases, a change in pressure will not affect equilibrium. Common-Ion Effect The solubility of CuCl in pure water is 1.1 10−3 M. The solubility of CuCl in sea water is 2.2 10−6 M. CuCl is 500 times less soluble in sea water. This dramatic reduction in solubility of CuCl demonstrates the common-ion effect. If you add chloride-rich ocean water to a saturated solution of copper(I) chloride, the [Cl−] increases. CuCl (s) Cu+(aq) + Cl-(aq) Common-Ion Effect Remember, Ksp = [Cu+] [Cl-] However, Ksp is a constant, so if the [Cl-] is increased, [Cu+] must decrease. This decrease can only occur by the precipitation of the CuCl salt. The ion Cl− is the common-ion in this case. Common-ion effect: the phenomenon in which the addition of an ion common to two solutes brings about precipitation or reduces ionization. You will see this when you add sodium citrate to a saturated NaCl solution. The NaCl will precipitate out. Practical Uses of Le Châtelier’s Principle The chemical industry makes use of Le Châtelier’s principle in the synthesis of ammonia by the Haber Process. High pressure is used to drive the following equilibrium to the right. The forward reaction converts 4 mol of gas into 2 mol of another gas, so it is favored at high pressures. Homework Section 14.3 review, pg. 518: #1-7 Le Châtelier’s principle worksheet Concept Review:”Equilibrium Systems and Stress” – we’ve now finished the chapter… Next time: Section 4.3 Quiz Chapter review work day Chapter 14 Test/Concept Review Due: Monday: April 21st: “A” Day Tuesday, April 22nd: “B” Day