Unit Title
advertisement

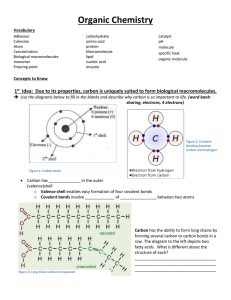
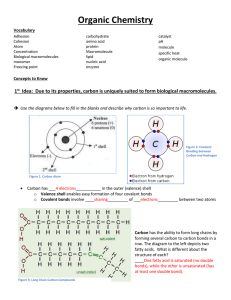
NAME________________ FINAL DUE DATE FOR ALL WORK: __________________________ _ GENERAL INSTRUCTIONS: 1. KEEP THIS PORTFOLIO IN A SAFE PLACE!!!!! I will be recording your scores on this page and nowhere else until I collect the portfolio. If you lose this sheet, you will need to produce the original work for regrading. 2. Each assignment has a point value based on the amount of time and effort necessary to complete that task. 3. I expect students to be working on this unit at all times while in the classroom. 4. If you complete more than the required work in any section, it will be considered as extra credit. Every assignment is valid and will increase your comprehension. 5. Notes will not be accepted after the due date, unless you have an excused absence. 6. Extra credit will not be accepted until all sections are completed. The Chemistry of Life Atom Element Compound Ionic Bond Ion Covalent Bond Molecule Hydrogen Bond Cohesion Adhesion Solution Solute Solvent pH acid base Monomer Polymer Carbohydrate Monosaccharide Lipid Nucleic Acid Nucleotide Protein Amino Acid Fatty acid Chemical Reaction Reactant Product Activation Energy Exothermic Endothermic Bond energy Equilibrium Catalyst Enzyme Substrate 1. All living things are based on atoms and their interactions. Compare and contrast ionic and covalent bonding. 2. Water’s unique properties allow life to exist on earth. Describe the unique properties of water and how these properties support life on Earth. 3. Carbon-based molecules are the foundation of life. Explain how carbon is uniquely suited to form biological macromolecules. Describe how biological macromolecules form from monomers. Compare the structure and function of carbohydrates, lipids, proteins, and nucleic acids in organisms. 4. Life depends on chemical reactions. Describe how living things use chemical reactions. 5. Enzymes are catalysts for chemical reactions in living things. Describe the role of an enzyme as a catalyst in regulating a specific biochemical reaction. Explain how factors such as pH, temperature, and concentration levels can affect enzyme function. NAME________________ The Chemistry of Life NOTES DUE DATE GRADE _________ Complete Outline/Notes on Ch 2.1 (10) __________ __________ _________ Complete Outline/Notes on Ch 2.2 (10) __________ __________ _________ Complete Outline/Notes on Ch 2.3 (21) __________ __________ _________ Complete Outline/Notes on Ch 2.4 (10) __________ __________ _________ Complete Outline/Notes on Ch 2.5 (10) __________ __________ NOTES TOTAL : _____ / 61 LABS _________ Water Lab (30) LAB TOTAL : _______ / TBD _________ To be Determined (TBD) REVIEW (complete at least 40 pts) _________ Answer the unit objectives (from the front of this paper) (40) _________ Reviewing Main Ideas: Complete pg. 61 #10 – 22 (48) _________ Critical Thinking: Complete pg. 62 #23-29 (28) _________ Interpreting Visuals: Complete pg. 62 #30-32 (12) _________ Analyzing Data: Complete pg. 62 #33-35 (12) _________ Connecting Concepts: Complete pg. 62 #36-37 (8) _________ Standards-Based Assessment: Complete pg. 63 #1 – 7 (10) REVIEW TOTAL : _______ / 40 CREATE (complete at least 1) _________ Model: Create a model of each of the four types of macromolecules . (40) _________ Mind-map: Create a mind-map poster of at least 20 of the vocabulary terms; be sure to include necessary supporting information such as definitions and draw connections of similar terms. (40) _________Diagram: Create posters for any eight vocabulary terms (each one on plain white paper, or four per regular sized poster paper) . (40) CREATE TOTAL : _______ / 40 Current Grade: ____% A B C D F