The Mole - Warren County Schools
advertisement

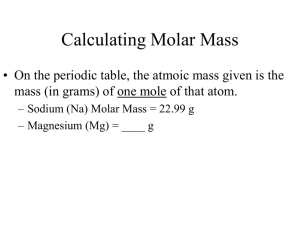
Moles are a Chemist Best Friend Chapter 10 nclark.net Objectives: Moles are a Chemist Best Friend • I can identify what a mole is in chemistry. • I can convert between representative particles of matter and moles. • I can convert between the mass of matter and moles. • I can convert between the volume of matter and moles. Chemistry (4/8) Infinite Campus Update: • Chemical Reaction Exam (raw avg. 62%) • Chemical Reaction Study Guide (10pts.)-optional Objectives: • Address Chemical Reaction Exam • Discuss Science Reflection Article • Identify what a mole is in chemistry. Homework: • Review Chemical Rxtn. Notes/Complete Study Guide • Science Reflection Article-due Friday • Need calculator for new unit- The Mole Science Reflection Article • Select one article to read on my webpage: a. “Drivers, Start Your (Electric) Engines!” b. “Green Gasoline: Fuel From Plants” • Complete annotation and evaluation questions. • Due: Friday, April 12th Chemical Reaction Exam • Chemical Exam Make-up Questions-Fri. Chemistry (4/9) Objectives: • Review endothermic vs. exothermic energy diagrams • Identify what a mole is in chemistry. Homework: • Mole Problems. (Bring calculator for this unit!) • Review chemical reaction notes/practice problems. • Science Reflection Article-due Friday • Chemical Reaction Make-up Qts-Friday Energy Diagram Does this represent an endothermic or exothermic reaction? (H) (time) Energy Diagram • • • H reactant > H product More energy will be released in this reaction Small activation energy (H) (time) Energy Diagram Does this represent an endothermic or exothermic reaction? Activation Energy (H) (time) Endothermic Energy Diagram • H reactant < H product •More energy is absorbed in this reaction •Large activation energy Activation Energy Chemistry (4/9) Objectives: • I can identify what a mole is in chemistry. • I can convert between representative particles of matter and moles. Homework: • Chemical Reaction Review-due Fri. • Science Reflection Article-due Fri. Moles are a Chemist Best Friend Chapter 10 nclark.net Objectives: Moles are a Chemist Best Friend • I can identify what a mole is in chemistry. • I can convert between representative particles of matter and moles. • I can convert between the mass of matter and moles. • I can convert between the volume of matter and moles. Measuring Matter What are three ways we can measure matter? 1. 2. 3. hermitwoods.com Measuring Apples www.123rf.com stockphotos.it flickr.com Grouping Matter • Easy way to measure all matter. r Gross of pencils= Dozen dougnuts= Ream of paper = Byte of stored information= Grouping Representative Particles • How would scientists group representative particles of matter? Representative particles: the substances that make-up matter. nclark.net Classification of Matter Grouping Representative Paraticles • How would we group representative particles that make-up matter? Representative particles: substances that make-up matter. (atoms, molecules or ionic compounds) nclark.net Grouping Representative Particles A mole is a unit of measurement that is used to group representative particles. 1 mole = 6.02x1023 representative particles (Avogadro’s Number) •“The Mole is a Unit” song (nclark.net) Mole Conversion Map http://mysite.cherokee.k12.ga.us Chemistry (4/10) Objectives: • I can identify what a mole is in chemistry. • I can convert between moles and representative particles of matter. Homework: • Mole Conversion Problems-due Thurs. • Chemical Reaction Review-due Fri. • Science Reflection Article-due Fri. Bell Ringer: Moles 1. a. What is a representative particle? b. Give two examples of representative particles. 2. a. What is a mole? b. How many atoms of Cu are in a mole of Cu? Mole Conversion Map http://mysite.cherokee.k12.ga.us Converting Moles to Representative Particles Ex. Propane (C3H8) is a gas used for cooking and heating. How many molecules of propane is equal to 2.4 moles of propane? Mole Conversion Map http://mysite.cherokee.k12.ga.us Convesion: Representative Particles to Moles Ex. Magnesium is a light metal used in the manufacture of aircraft and automobile wheels. How many moles of magnesium equals 1.25x1023 atoms of magnesium? Mole Conversion Map http://mysite.cherokee.k12.ga.us Conversion: Mole-Representative Particles Homework Problems A Mole is a Chemist Best Fried wks. When calculating mole conversions…. • Show work so errors can be identified and corrected. • Multiply what your given by the conversion fraction. They are NOT equal or proportional to one another. • If dividing scientific notation values, place parenthesis around the value in the denominator when plugging in calculator. • Express answer with correct number of significant figures (sigs.); units. Chemistry (4/11) Objectives: • I can convert between moles and representative particles of matter. • I can convert between moles and mass of matter. Homework: • Mole Conversion Problems-Fri. • Chemical Reaction Review-due Fri. (optional) • Science Reflection Article-postpone Mon. Friday: Chemical Reaction Make-up Qts. (16qts ) Conversion: Mole-Representative Particles Homework Problems Converting Moles to Mass • How do we convert from moles to mass of a substance? Mole Conversion Map http://mysite.cherokee.k12.ga.us Conversion: Moles- Mass • Mole-Mass Conversion: 1 mole of a substance = molar mass of the substance Molar Mass of an Element • molar mass of element = atomic mass of element • Round atomic mass to tenths place. Ex. A mole of sulfur (S) has a molar mass of? Ex. A mole of sodium (Na) has a molar mass of? Molar Mass of a Compound • molar mass of compound = sum of the elements’ atomic masses in the compound. • Need chemical formula to calculate the molar mass of a compound. Ex. A mole of NaCl has a molar mass of? Ex. A mole of H2O has a molar mass of? Molar Mass of Compounds Mole-Mass Conversions Ex. How many moles are in 2.5 grams of Cu? Ex. How many grams are in 3.8 moles of FeO? Chemistry (4/15) Due Today: • Science Reflection Article qts. Infinite Campus Update: • Chemical Reaction Make-up Qts.(optional) • Moles are a Chemist’s Best Friend Wks. (5pts.) Objectives: • Calculate molar mass of a substance. • Convert between moles and mass of a substance. Homework: • Mole Packet Bell Ringer: Chemistry 1. What is the representative particle for each substance below? (atom, ionic compound, or molecule) a. Na2O b. Fe c. H2 d. Ne 2. How many atoms are in 2.5 moles of copper? 3.a. What is the molar mass of silver, Ag? b. What is the molar mass of Al2(CO3)3? Chemistry (4/16) Objectives: • Calculate molar mass of a substance. • Convert between moles and mass of a substance. Homework: • Mole Conversion Worksheet *Quiz: Friday -mole conversions Conversions: Mole-Mass Ex. How many moles of iron (III) oxide are contained in 92.2g of pure Fe2O3? Ex. How many grams are in 2.68 moles of Fe2O3? Mole-Mass Conversions Conversions: Mole-Mass • 1 mole = molar mass of substance Ex. When iron is exposed to air, it corrodes to form red-brown rust. How many moles Fe2O3 are contained in 92.2g of pure Fe2O3? Chemistry (4/17) Objective: • Discuss qts. over homework (mole conversions) • Mole Conversion Lab Homework: • Complete Mole Conversion Lab post questions. Mole Conversions Mole Conversion Lab Purpose: Practice mole conversions with substances in the lab. Hypothesis: How many particles are in a tablespoon of each of the following substances? • H2O • Ca(CO3) • NaCl Mole Conversion Map http://mysite.cherokee.k12.ga.us Chemistry (4/18) Objective: • Calculate 2-Step Mole Conversions • Address questions over Mole Conversion Lab Homework: • 2-Step Mole Conversion Problems *Quiz tomorrow: mole conversions 2-Step Mole Conversions Ex. How many grams are there in 2.4x1013 atoms of Carbon? Ex. How many molecules are in 48.4 grams of Ca(PO4)2 ? Mole Conversion Map http://mysite.cherokee.k12.ga.us Chemistry (4/19) Objective: • Discuss 2-Step Mole Conversion Problems (hmwk) • Mole Conversion Quiz • Calculate % Composition of a Compound Homework: • % Composition of a Compound • Chemical Quantities Study Guide *Chemical Quantities Exam: Wed., April 19th 2-Step Mole Conversions-Key 3. 1.3x1023 atoms 4. 1.0x1024 molecules 5. 410 grams 6. 210 grams 7. 120 grams 8. 3.92x1023 molecules 9. 310 grams 10. 1.1x1024 Chemistry (4/22) Objective: • Calculate % Composition of a Compound • Discuss Mole Conversions • Take Mole Conversion Quiz Homework: • % Composition of a Compound worksheet and lab. • Chemical Quantities Study Guide *Chemical Quantities Exam: Wed/Thurs., April 24th/25th Chemistry (4/22) Objective: • Calculate % Composition of a Compound • Complete Mole Lab • Discuss Mole Conversion Quiz and Study Guide Homework: • % Composition of a Compound worksheet and lab. • Chemical Quantities Study Guide *Chemical Quantities Exam: Wed., April 24th Chemistry (4/22) Infinite Campus Update: • Mole Conversion Worksheet (10pts.) • Science Article Questions (15pts.) Midterm Grades Posted: 4/24 Percentage Composition of a Compound Percentage Composition of a Compound • % by mass of each element in a compound • How do we calculate % composition of a compound? Percentage Composition of a Compound Percentage Composition of a Compound • % by mass of each element in a compound What is the percent composition of NaCl? Chemistry (4/23) Objectives: • Review for Chemical Quantities Exam *Chemical Quantities Exam : April 24th/25th Homework: Complete study guide-key on my webpage Chemistry (4/23) Objectives: • Review for Chemical Quantities Exam *Chemical Quantities Exam : April 24th (tomorrow) Homework: Complete study guide-key on my webpage Chemical Quantities: Bell Ringer 1. How many moles are in 7.4 grams of Ba(OH)2? 2. How many grams are in 3.8x1019 atoms of C? 3. Calculate the % composition of Al(NO3)3. 4. How many grams of N are in 5.4 grams of Al(NO3)3? Chemical Quantities: Bell Ringer 1. How many moles are in 7.4 grams of Ba(OH)2? 2. How many grams are in 3.8x1019 atoms of C? 3. Calculate the % composition of Al(NO3)3. 4. How many grams of N are in 5.4 grams of Al(NO3)3? Chemistry (4/24) Infinite Campus: • Mole Lab (15pts.) • Mole Worksheet (10pts) • Science Article Qts. (15pts.) Objectives: • Chemical Quantities Test • Introduce Hydrate Lab Tomorrow: Hydrate Lab Percent Composition of Compound Percentage Composition of a Compound Percentage Composition of a Compound • % by mass of each element in a compound • How many grams of Na are in a 3.5 gram sample of NaCl? • How many grams of Cl are in a 20 gram sample of NaCl? Mole Quiz Study Guide % Composition from Chemical Formula % mass of element = Ex. Propane, C3H8 is one of the compounds obtained from petroleum and is used to fuel homes and gas grills. Calculate the percent composition of propane. % Composition as a Conversion Factor • Use % composition data to calculate the mass of an element in a specific sample of that compound. • Ex. What is the mass of Al in 6.5 g of Al(NO2)3 ? % Composition as a Conversion Factor •What is the mass of Al in 6.5 g of Al(NO2)3 ? % Composition as a Conversion Factor •What is the mass of N in 6.5 g of Al(NO2)3 ? •What is the mass of O in 6.5 g of Al(NO2)3? Bell Ringer: Chemical Quantities 1. How many grams are in 5.0 moles of Cs3N ? 2.How many moles are in 3.7x1021 cmpds. of NaCl? 3. What is the % composition of Cu2(SO4)? 4. How many grams of Cu are in 9.8 g of Cu2(SO4)? Empirical vs. Molecular Formulas Molecular: Actual whole-number ratio of atoms in compound. Ex. Glucose molecular formula= C6H12O6 Empirical: Smallest whole-number ratio of atoms in a compound. Ex. Glucose empirical formula = CH2O Molecular H2O2 (hydrogen peroxide, antiseptic) C2H2 (fuel used in welding) C6H6 (styrofoam) N2O4 (rocket propellant) CO2 (carbon dioxide) Empirical What is the molar mass of Cr3N2? • How many moles of FeO are in 5.4x1026 compounds of FeO? How many grams of Be3(PO3)2 are in 7.84 moles of Be3(PO3)2 ? How many atoms of Br are in 3.6 moles of Br? Empirical vs. Molecular Formulas Chemical Name CH C2H2 (acetylene) C6H6 (benzene) CH2O (methanol) C2H4O2 (ethanoic acid) C6H12O6 (glucose) Formula Classification Calculate Empirical Formula • Use % composition data and molar mass of elements . Ex. A compound is analyzed and found to contain 25.9% N and 74.1% O. What is its empirical formula? 25.0g N x ( 1mole N/14gN ) = 1.85 moles N 74.1 g O x (1moleO/16 g O) = 4.63 moles O Numbering Matter What are some familiar terms we use to number matter? Moles Representation • A mole can be used to represent anything. • Ex. A mole of moles = 6.02x10 23 moles of moles Bell Ringer 1. What do the following terms have in common? pair, dozen, byte, and mole? 2. What does 1 mole equal? Is it a big or small value? 3. Give a practical example using the term mole. Ex. 1 mole of carbon atoms. Mole and Particles 1 mole = 6.02x1023 particles (atoms, ions, or compounds) • How many Li atoms are in 4.0 moles? • How many Li1+ ions are in 2.0 moles? • How many moles are in 1.35x1020 of H2O molecules? Where did they get Avogadro’s Number? • Scientist discovered that 12g of Carbon was equal to one mole. 12 g = molar mass of C • Scientist discovered that 35g of Chlorine was also equal to one mole. 35g = molar mass of Cl • Is there a chemistry term we can associate with molar mass? Molar Mass • Molar mass is the mass of an element that equals 1 mole. Cu(SO4) . Hydrate Lab H2O ----> Cu(SO4) + H2O Use the equation above to help answer the following questions: 1. What is the hydrate salt in this equation? 2. What is the anhydrous salt in this equation? 3. What is the difference between a hydrated salt and an anhydrous salt? 4. Is this a physical or chemical reaction? 5. What did you calculate the chemical formula for the hydrated salt to be? Copper Sulfate Hydrate Mole Map mass molar mass 1mol 1mol molar mass moles 22.4L 1mol 1mol 6 .02 x 10 23 part icles 1mol 22.4L 6 .02 x 10 23 part icles 1mol volume at STP number of represent at ive part icles Mole and Mass Conversion • 1 mole of Mg is equal to how many grams of Mg? • 4 moles of N2 is equal to how many grams of N2? • 2.5 moles of CH4 is equal to how many grams of CH4? Mole and Mass Conversions • How many moles is equal to 172 g of NaCl?