Summative Matter Unit Test Study Guide (w
advertisement

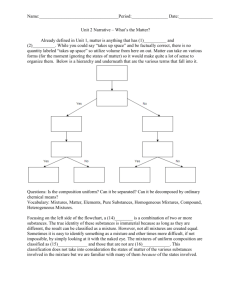
Summative Matter Unit Test Study Guide (w/answers) 1. Why is it important to control variables in an experiment? - It is important to control variables in an experiment to insure a “fair test”. There should only be 1 independent variable being tested in an experiment. 2. What is matter? (p.38) Matter is anything that has mass and takes up space. 3. What is the Metric System? (p.25) The metric system is a system of measurement that is used by scientists all over the world. Most countries only use the metric system. 4. What is mass? Mass is the amount of matter, or atoms in an object. 5. What units are used to measure mass? (p.25). Grams (can be used with any metric prefix) kilograms, hectograms, dekagrams, decigrams, centigrams, milligrams 6. What is the difference between mass and weight? (p.41) Mass is the amount of matter, or atoms an object has; mass is constant (never changes). Weight is the measure of the gravitational force exerted on an object; weight can change depending on your location in the universe. 7. What is volume? (p.25-26 & 39-40) Volume is the amount of space an object takes up or occupies. 8. What units would be used to measure the volume of a solid? The volume of a solid would be recorded in cm3. 9. What units would be used to measure the volume of a liquid? (p.25-26 & 39-40) The volume of a liquid would be recorded in ml or liters. 10. List 6 physical properties of matter? (p.44-45) -malleability - thermal conductivity -ductility -solubility -state -density 11. What is density? (p.46-47) Density is the amount of mass of a substance in a given space or volume. 12. What is formula for density? (p.46-47) D = Mass/Volume 13. What would the units of density be for a solid? (p.46-47) g/cm3 Summative Matter Unit Test Study Guide (w/answers) 14. What would be the units of density for a liquid? (p.46-47) g/ml 15. List the 3 chemical properties of matter? (p.50-51) - Combustibility - Flammability - Reactivity 16. Why are chemical properties harder to observe than physical properties? (p.51) Chemical properties are harder to observe than physical properties because in order to observe an object’s flammability, reactivity, or combustibility – the object must burn, react, or explode. 17. What is the difference between a physical change and a chemical change? (p.54-55) A physical change usually involves a change in appearance or some other superficial alteration. A chemical change involves a change in the identity and properties of a substance. 18. List all of the signs of a chemical change? (p.53) - Fizzing/foaming - Production of sound - Production of heat - Production of light - Odor/smell - Change in color 19. What are the four states of matter? (p.66-69) - Solid - Liquid - Gas - Plasma 20. Explain how the particles move in each state of matter. (p.66-69) - Solid (particles vibrate in place) - Liquid (particles slide past each other) - Gas (particles are very far apart, move quickly and randomly) - Plasma (CHARGED particles are very far apart, move quickly and randomly 21. Which state of matter has the particles that move the fastest? (p.66-69) Plasma > gas > liquid > solid (speed of particles) 22. What state of matter has the greatest amount of energy? (p.66-69) Plasma > gas > liquid > solid (energy of particles) 23. What is the difference between a gas and plasma? (p.66-69) Gas particles and plasma particles both move quickly and randomly with no definite shape or volume. Plasma particles move faster than gas particles because they have a higher temperature and plasma particles are charged (negative and positive). Summative Matter Unit Test Study Guide (w/answers) 24. List all the changes of state and define them (p.74-78) - Melting (Solid To Liquid) - Freezing (Liquid To Solid) - Condensation (Gas To Liquid) -Evaporation (Liquid To Gas) - Sublimation (Solid To Gas) - Boiling (Liquid To A Vapor) 25. Define closed system. A closed system is a contained environment for chemical reactions to take place without gaining or losing energy and/or mass. 26. What is an atom? (p.318-321) • AN ATOM IS THE SMALLEST UNIT OF MATTER • IS THE “BUILDING BLOCK “OF ALL ELEMENTS • HAS THE SAME PROPERTIES AS THE ENTIRE ELEMENT 27. Draw and label an atom with the particles.(p.319) e- P+ N0 28. What charge does each particle have? (p.319-321) Proton – positive (+) Neutron – neutron (0) Electron – negative (-) 29. If element X has an atomic number of 15 and atomic mass of 31.65, how many protons, neutrons and electrons does the atom have? Protons = 15 Neutrons = 17 Electrons = 15 30. What is a pure substance? What 2 types of matter are pure substances? (p.91 & 94) - substance in which all the particles look the same Summative Matter Unit Test Study Guide (w/answers) - pure substances can only be elements or compounds 31. What is an element? (p.90) - AN ELEMENT IS A SUBSTANCE THAT CANNOT BE BROKEN DOWN INTO SIMPLER SUBSTANCES BY PHYSICAL OR CHEMICAL MEANS. 32. How is the periodic table arranged? (p.336-342) The periodic table is arranged in groups (columns) and periods (rows) according to their atomic number (number of protons) and categorized further by trends such as electrons in outer shell, characteristic properties, metals, nonmetals, and metalloids. 33. What is a group/family? - The different columns of elements are called groups or families – Elements in the same family have similar properties 34. What is a period? (P.336-342) - The different rows of elements are called periods. – The period number of an element signifies the highest energy level an electron in that element occupies 35. How do properties change as you move across a period? (P.336-342) Properties such as conductivity and reactivity change gradually as you move across a period (elements become less reactive and less metallic) 36. Explain why the elements in a group/family are more closely related than those in a period? Elements in a group/family are more closely related than those in a period because they share the same number of electrons in their outer shells and are able to bond or react with the same types of elements. 37. What is a compound? • A chemical compound is a chemical substance formed from two or more elements in a specific ratio. 38. How are compounds formed? (p.94-97) Compounds form in a specific ratio according to their position on the periodic table 39. What happens to the identities of the elements when a compound is formed? (p.94-97) • The elements lose their individual chemical properties and the compound has new properties. 40. Give 3 examples of chemical formulas. H2O – Water NaCl – Table Salt CH3COOH – Vinegar O2 – oxygen CO2 – Carbon Dioxide C – Carbon 41. Compare and contrast compounds and mixtures? (p.96) COMPOUNDS A pure substance containing two or more kinds of atoms MIXTURES Two or more elements or compounds NOT chemically combined. Summative Matter Unit Test Study Guide (w/answers) The atoms are chemically combined in some way. Often times (but not always) they come together to form groups of atoms called molecules. No reaction between substances. Compounds cannotbe separated by physical means. Separating a compound requires a chemical reaction. Mixtures can be uniform (called homogeneous) and are known as solutions. Mixtures can also be non-uniform (called heterogeneous) Mixtures can be separated into their components by chemical or physical means. The properties of a compound are usually different than the properties of the elements it contains. 42. What is a solution? (p.98-99) • A solution is a homogeneous mixture in which all substances are well mixed and appear to be one substance throughout. 43. What is the difference between a solution (homogeneous mixture) and a heterogeneous mixture? (p.98) A homogeneous mixture (solution) appears to be one substance throughout and very well mixed. The particles are too small to be identified but can still be separated physically. A heterogeneous mixture is not very well mixed and substances are easily identified because they retain their appearance and properties. 44. In a chemical reaction, what is the reactant? What is the product? Reactant – The starting materials in a chemical reaction; a substance or material that participates in a reaction Product – The substance that forms in a chemical reaction C + Reactants O2 CO2 Product 45. What does the law of conservation of mass mean? Matter, or mass cannot be created or destroyed. It can only change forms in a chemical or physical change. Atoms rearrange themselves. For example, in any chemical reaction or change, the mass of the reactants, or starting materials, is equal to the mass of the product. Example: 2 slices of bread + 1 tablespoon-Peanut Butter + 1 tablespoon-Jelly (30 g) + (14 g) + (14 g) = PB&J sandwich (58 g)