Unit 3 Review #2 unit_3_compounds_review
advertisement
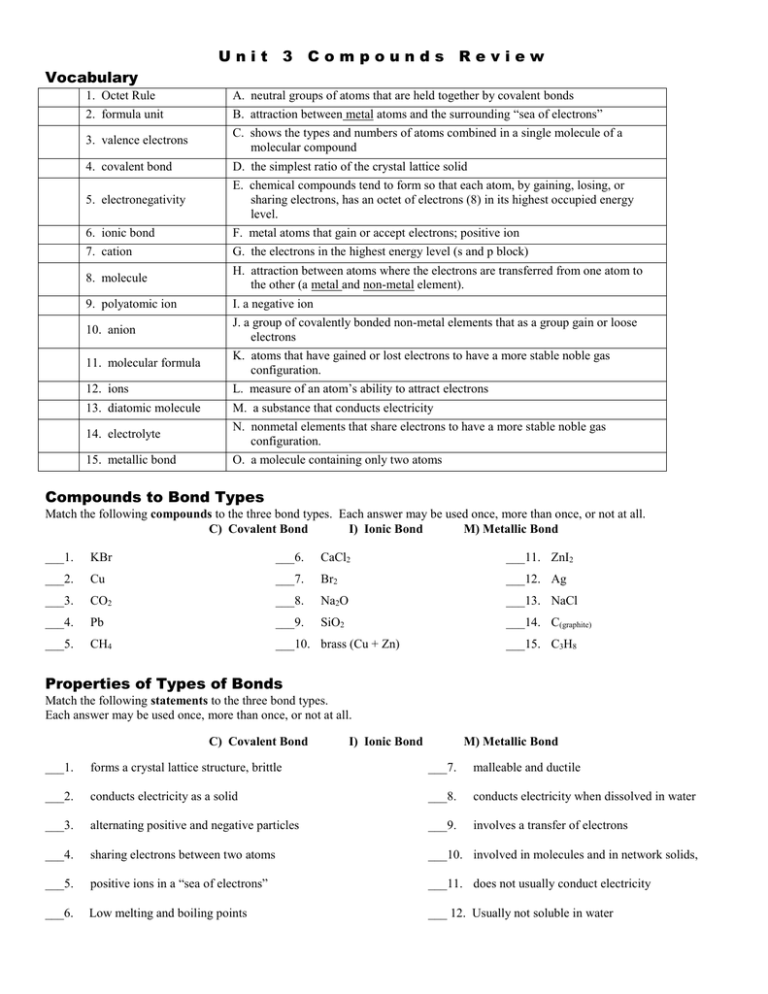
Unit 3 Compounds Review Vocabulary 1. Octet Rule A. neutral groups of atoms that are held together by covalent bonds 2. formula unit B. attraction between metal atoms and the surrounding “sea of electrons” 3. valence electrons C. shows the types and numbers of atoms combined in a single molecule of a molecular compound 4. covalent bond D. the simplest ratio of the crystal lattice solid 5. electronegativity E. chemical compounds tend to form so that each atom, by gaining, losing, or sharing electrons, has an octet of electrons (8) in its highest occupied energy level. 6. ionic bond F. metal atoms that gain or accept electrons; positive ion 7. cation G. the electrons in the highest energy level (s and p block) 8. molecule H. attraction between atoms where the electrons are transferred from one atom to the other (a metal and non-metal element). 9. polyatomic ion I. a negative ion 10. anion J. a group of covalently bonded non-metal elements that as a group gain or loose electrons 11. molecular formula K. atoms that have gained or lost electrons to have a more stable noble gas configuration. 12. ions L. measure of an atom’s ability to attract electrons 13. diatomic molecule M. a substance that conducts electricity 14. electrolyte N. nonmetal elements that share electrons to have a more stable noble gas configuration. 15. metallic bond O. a molecule containing only two atoms Compounds to Bond Types Match the following compounds to the three bond types. Each answer may be used once, more than once, or not at all. C) Covalent Bond I) Ionic Bond M) Metallic Bond ___1. KBr ___6. CaCl2 ___11. ZnI2 ___2. Cu ___7. Br2 ___12. Ag ___3. CO2 ___8. Na2O ___13. NaCl ___4. Pb ___9. SiO2 ___14. C(graphite) ___5. CH4 ___10. brass (Cu + Zn) ___15. C3H8 Properties of Types of Bonds Match the following statements to the three bond types. Each answer may be used once, more than once, or not at all. C) Covalent Bond I) Ionic Bond M) Metallic Bond ___1. forms a crystal lattice structure, brittle ___7. malleable and ductile ___2. conducts electricity as a solid ___8. conducts electricity when dissolved in water ___3. alternating positive and negative particles ___9. involves a transfer of electrons ___4. sharing electrons between two atoms ___10. involved in molecules and in network solids, ___5. positive ions in a “sea of electrons” ___11. does not usually conduct electricity ___6. Low melting and boiling points ___ 12. Usually not soluble in water Lewis Dots of Elements & Ions Draw Lewis Dot Symbols of the following elements and ions: Na Cl Na K+ O Cl FK I O S2F Al I BrS Al Ca2+ Br Ca Lewis Diagrams and Polarity 1. 2. 3. Consider the following Lewis Dot Symbols. Circle the elements (and electrons) that follow the octet rule. State whether each molecule below is polar or non-polar. Explain to each other why you made your choice. Which compounds have double or triple bonds? Example: Carbon dioxide Electronegativity difference, but cancels out. Nonpolar Lewis Dots of Molecules and Polarity 1. 2. Draw the Lewis Symbols of the following molecules. Only single bonds are used. State whether each molecule below is polar or non-polar. O2 CH3Cl H2O2 H2O PH3 SiH4 CH3COOH C2H6 CH3OH Shape, Bond Angle, and Polarity VSEPR Shapes: The shape and bond angle of a molecule is based on the number of bonds and pairs of lone electrons off the central atom. Use the Lewis Structures above to determine the shape, bond angle, and polarity of each molecule. Polarity: Shape of Molecule (central atom) Bond Angle Polarity Compounds Bonds Lone Pairs Shape O2 CH3Cl H2O2 H2O PH3 SiH4 CH3COOH C2H2 CH3OH Names and Formula - Covalent Compounds Covalent compounds two or more _____________________ elements that __________________ 1. 6. 2. 7. 3. 8. 4. 9. 5. 10. electrons to complete an octet of valence electrons. Covalent compounds are also called _______________________. Rules: 1. Use _________________ to indicate the number of each kind of atom. 2. Add the ______________ ending to the second element. 3. Do not use ____________________ on the first element in the compound name. Write the name and the formula for the following covalent compounds. Covalent Names Covalent Formula 1. SO2 1. disulfur hexachloride 2. P3Br4 2. dinitrogen pentoxide 3. N2O5 3. carbon tetriodide 4. CCl4 4. tetraphophorus dekoxide 5. P2I7 5. phosphorus trichloride 6. ClO2 6. trisulfur octoxide 7. SF6 7. nitrogen monoxide Ionic Compounds Ion Charge The forces of attraction between oppositely charged ions in the compounds are called ____________. Atoms that have given up electrons and are positive are charge is called a ___________. Atoms that have gained electrons and are negative in charge are called ______________. Ionic compounds form _______________ ____________________ where oppositely charged ions line up next to one another to minimize repulsion forces The representative particle is a _____________________. Ionic Compound Formula Rules: 1. Write the charge of each ion. 2. Determine the correct number of each ion needed to have an overall charge of zero. 3. Write the number of each ion by adding subscripts. # Cations Cation 3 NH4+ # Anions 1 Anion 3- PO4 Ba2+ NO3- Al3+ CO32- Sr2+ OH- Fe3+ C2H3O2- Compounds Iron (II) chloride Sodium chloride Copper (II) oxide Magnesium oxide Calcium bromide Lead (II) sulfate Aluminum oxide cobalt (II) nitrate FeF3 Na2O K2SO4 (NH4)3N MnO2 Cr2 (SO4)3 Formula (NH4)3PO4 Group A Metal? Compound name ends in -ide Write and balance charges More than 2 different elements present? Transition metal? Roman #? Compound ends in -ide Roman number – charge of metal atom Polyatomic ion (-ate or –ite) 1. Identify polyatomic ion. 2. Write and balance charges Percent Composition Calculate the molar masses of NaOCl and Ca(NO3)2. Calculate the percent composition of each element in the following compounds. NaOCl Na = = O = = Cl = = Ca(NO3)2 Ca = = N = = O = = Empirical and Molecular Formula Empirical formula: the lowest whole number ratio of atoms in a compound. Molecular formula: the true number of atoms of each element in the formula of a compound. Empirical HO CH NO2 Molecular H2O2 C2H2 N2O4 Given that the empirical formula of a compound is CH and the molar mass is 104 g/mol, calculate the molecular formula. 1. Find the mass of the empirical formula. 2. Divide the molar mass by the mass of the empirical formula. 3. Multiply the subscripts in the empirical formula by the difference (answer to #2). Given that a certain compound is 69.58% Ba, 6.090% C and 24.32% O, calculate the empirical formula of this compound. 1. Assume that you have 100.00 g of the compound: 69.58% Ba = 69.58 g Ba 6.090% C = 6.090 g C 24.32% O = 24.32 g O 2. Convert the mass of each element to moles of that element: 3. Divide through each value by the smallest number of moles to get a ____________________:_________________ ratio, which rounds of nicely to give the formula ___________________________. Names and Formula for Acids Write the formula of the acid formed from the following anions. Anion acid formula anion acid formula ClSO42OHPO432S C2H3O2- anion MnO4FNO3- acid formula Name the following acids: formula acid name HBr HNO3 HC2H3O2 formula H2CO3 H2S H3PO4 acid name formula HI H2Cr2O7 HClO3 acid name Given the anion, write the formula of the acid and then name it: Anion formula acid name S2ClNO3C2H3O2- anion SO42ClO3FCO32- formula acid name Moles – Mass- Particles - Volume The __________________ is the amount of substance that contains as many particles as there are in _______________________. The amount of substance that contains the _____________________ number of particles (1 mol = 6.02 x 1023 particles). Particles can be many units; elements are measured as ________________, covalent compounds are counted as ________________, and ionic compounds are counted as _______________ ____________. One mole of any gas will occupy ______________ liters at standard temperature and pressure (STP). Moles and Mass Conversions The molar mass is the mass of 1 mole or 6.02 x 10 23 atoms, molecules, or formula units. The molar mass for single elements is found on the _____________ _________________. The mass unit for a single atom is Mole and Mass Relationships 1. Converting moles to mass Amount of moles x Molar Mass 1 mole given 2. Converting particles to moles Given mass x 1 mole given Molar mass = mass grams (grams) = moles given ___________ ________________ _____________ (AMU). The mass unit for one mole of an element or compound is measured in _________________. 1. How many grams are in 2.5 moles of sodium carbonate, Na2CO3? 2. What is the mass of 1.25 moles of hydrogen gas, H2? 3. How many moles are 48.5 grams of sodium chloride, NaCl? 4. How many moles are in 96 grams of glucose, C6H12O6? Moles and Molecule Conversions The amount of substance that contains the _____________________ number of particles (1 mol = 6.02 x 1023 particles). Particles can be many units; elements are measured as ________________, covalent compounds are counted as ________________, and ionic compounds are counted as _______________ ____________. 1. Determine the number of moles in 2.4 x 10 22 molecules hydrogen gas, H2? 2. How many moles of water are in 4.5 x 10 24 molecules water, H2O? Mass and Molecule Conversions 1. How many molecules of glucose are in 96 grams, C6H12O6? 2. What is the mass of 1.25 moles of hydrogen gas, H2? 3. How many formula units are 48.5 grams of sodium chloride, NaCl? Mole-Particle Relationships 1. Converting moles to particles Amount of moles x 6.02 x 1023 particles = particles given 1 mole given 2. Converting particles to moles Given particles x 1 mole given = moles given 6.02 x 1023 particles





