FoodCalorieLab (1) - Freeman Public Schools
advertisement

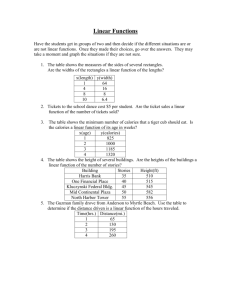
Calorie Lab Question: How many calories are there in Cheetos Puffs, a Rice Krispie Treat, Planters Peanuts, Lay’s Potato Chips, Fritos, and Doritos? The purpose of my lab is to compare the number of calories I found in my experiment to the number of calories in a package and determine percent error. Research: There are two types calories, a lowercase calorie and an uppercase Calorie. A lowercase calorie is the amount of energy needed to raise the temperature of one gram of water one degree Celsius. An uppercase Calorie, or a kilocalorie, is the amount of energy needed to raise the temperature of one kilogram of water one degree Celsius. In this lab report, I will refer to the uppercase Calorie which is also called the food calorie. One Calorie is equal to 1000 calories and one calorie is equal to 4.2 Joules. A Joule is another way of measuring energy. The calorie was first defined by Nicolas Clement in 1824, as a unit of heat. The word itself comes from the latin word ‘calor’ meaning heat. However in the United States today, we refer to calories as the amount of energy inside of food items. Everywhere else in the world uses joules instead of calories, similar to how we use inches and everyone else uses centimeters. In 1990, the Nutrition Labeling and Education Act (NLEA) was passed as stated by todayifoundout.com. This required all packaged foods to have a nutrition label and all health claims for foods to be consistent with terms defined by the Secretary of Health and Human Services. This act was the first act that required the nutrition label we know today. Today, the FDA requires that all food companies print accurate calorie counts on nutrition labels for every single product. This helps make it clear to consumers how many calories are consumed in an individual serving. The FDA also requires the amount of calories that come from fat alone. Calories are needed to provide energy so the body functions properly. The number of calories in a food or drink depends on the amount of energy the food or drink provides. Many foods and drinks have different amounts of energy-containing nutrients or proteins, carbohydrates, fats, and alcohol in them. According to kidshealth.com, in one gram of protein there are 4 calories, in one gram of carbohydrates there are also 4 calories, in one gram of fat there are 9 calories, and in one gram of alcohol there are 7 grams. This is why many fatty foods like chips, popcorn and french fries have a lot of calories in them. For example, lettuce, not a fatty food, has 5 calories in a cup or .14 calories per gram, but Doritos, a fatty food, has 150 calories in an ounce or 5.36 calories per gram. In my lab, I will not be able to use water-based items like lettuce, but will have to use food items like Doritos to be able to burn the food. Calories can be measured by two primary ways: the bomb calorimeter and the Atwater system according to symptomfind.com. In the bomb calorimeter model, food is placed inside a sealed container called a bomb calorimeter. Water surrounds the container and when the food has been burned, the rise in water temperature is measured to determine the amount of calories. The calorie measurement methods today use the Atwater system, which is USDA regulated. The Atwater system determines the energy-containing nutrients in the food or the amounts of the proteins, carbohydrates,fats, and alcohol. These amounts are totaled up and combined to give a food’s calorie total. Even though the USDA’s uses the Atwater System, some food companies use highly sophisticated, modern calorimeters to make sure the USDA’s calorie counts are correct. The average person should consume about 2,000 to 2,500 calories per day according articles.latimes.com. The number of calories a person needs depends on a person’s age, height, weight, gender, and activity level. People can gain and lose weight by watching their calorie intake. To lose weight, you should eat less calories than you take in and likewise if you want to gain weight, you should eat more calories than you burn off in normal daily activity or during exercise. In a pound of fat, there are 3,500 calories. So, if you want to lose a pound, you need to burn 3,500 calories. In my lab, I will use the Q = M *C *∆T to measure the number of calories. The symbols in the equation are: amount of energy(Q), amount of mass(M), specific heat of the substance(C), change in Temperature(∆T). The formula will be used to find the number of experimental calories and compare them to the number of actual calories and find the percent error. I will find the number of actual calories by finding the number of calories per gram and multiplying it by the mass burned from the food items. I will do this because the food items won’t burn completely. The energy of the burnt food will be used to heat the water according to the Law of Conservation of Energy. I will measure this change of temperature to find the calories. In my group’s experiment, we will use a calorimeter. Our calorimeter will be in an upsidedown, large tin can with the food burned under a smaller can that is elevated by two popsicle sticks. The smaller can will be elevated by the popsicle sticks because the smaller can has a lip that will rest on the popsicle sticks. The food will be placed on a regular tin can that will be cut for easy access to the food. Our group will have holes in the top of our lid to allow smoke to escape. The large tin can will be wrapped in tin foil to not allow heat to escape our large tin can. Our group will have the large tin elevated 2 cm off the ground to allow oxygen for the fire. Hypothesis: The Planters Peanuts will have the least amount of percent error, because the Planters Peanuts are the most dense and oily, and will be able to heat up the water and be the most accurate. Next, the Doritos will have the second least amount of percent error because they will be easy to start on fire because they are flat and will be able to burn the longest. The Rice Krispie Treats will have the third least amount of percent error because a Rice Krispie Treat is packed together pretty well and will burn a more completely. The Lay’s Potato Chip will have the fourth least amount of percent error because it will burn slower than the Cheetos Puff and will be easier to start than the Fritos. The Cheetos Puff will have the fifth least amount of percent error because the Cheetos Puff will be easier to burn than the Fritos, because it has more surface area than the Fritos to start on fire. Lastly, the Fritos will have the most percent error because it will be harder to start on fire than the other foods. Experiment: Safety: -Wear safety glasses when you are burning and when using the dremel to prevent smoke from getting in your eyes while burning and to prevent tin can scraps from getting in your eyes when using the dremel. -Use hot hands when handling the hot can with a lip to prevent getting burnt. -Use a fume hood when burning so the smoke doesn’t fill the room. -Make sure hair is pulled back so it doesn’t catch on fire when burning. Materials: -One large tin can about 15 cm in diameter and 18 cm tall -One smaller can about 6.75 cm in diameter with a .25 cm lip -Regular tin can about 7 cm in diameter -2 popsicle sticks -Gram scale -Water either distilled or tap -1 Paper clip -Nail with a 1 cm diameter -Hammer -Dremel -Box knife -Tin foil -Food Samples: Planters Peanuts, Cheetos Puffs, Rice Krispie Treats, Doritos, Fritos, and Lay’s Potato Chips -Thermometer -Hot Hands -Goggles -Access to a fume hood -Lighter -Five pieces of long kindling to stick in the door of your large tin so you can light your food Procedures: 1. Get your large tin can and flip it upside down. 2. Use your dremel and cut 4 slits 13 cm up the side of your large tin can. The slits should be vertical and be .25 cm wide and 1.5 cm long to fit the popsicle sticks inside them. The slits should be 6.5 cm away from each other. 3. Use the dremel to create a 2.5 cm by 3.5 cm box about 1 cm up the side of the large tin can. 4. Put the two popsicle sticks in their slits parallel to each other. 5. Use the hammer and nail to make seven holes in a triangle formation holes in the center of the top of the can. The holes should be about 4 cm apart from each other. The holes should be wide enough to be able to put your thermometer in them. The holes are to let the smoke out and the Oxygen in. 6. Grab your box knife and regular tin can. Cut the tin can 3 cm from the base of the can so that your can is only 3 cm tall. This is what you will set your food on to burn. 7. Cut a 3 cm door in your regular tin can. 8. Set the regular tin can under, and in the center of, the large tin can with the popsicle sticks inside. Put the small can so that the door of the small can is facing the door of the large tin can, so you can use your this wood stripes to light the food. 9. Depending on the type of food burning, you can prop it up or hold it on a paper clip to make the food easier to light. For example, I stuck the paper clip through my Planters Peanuts so I could burn underneath them. I learned that the foods burn more completely when you burn them from the bottom up. 10. Wrap your large tin can in tin foil, then when you wrap the top of the can, you will need to make the seven holes again through the tin foil. You will also need to make the door of the large tin can again through the tin foil. I will wrap the large tin can in tin foil so the heat is not allowed to escape. 11. Put your amount of water in your can with a lip, you should have at least 150 mL of water, you might want to add more water to prevent the water from boiling. In my experiment, I will use 150 mL and 200 mL of water. 12. Put your smaller can with a lip between your parallel popsicle sticks. The lip of the can should set of the two popsicle sticks. 13. Stick your thermometer through the center hole of your large can. Make sure the thermometer is far enough down so it is inside the water of the can with a lip. 14. Set your food inside of them regular tin can that is 3cm tall. Make sure you record the initial Mass of the food sample and initial Temperature of the water. 15. With your food under the large tin can, you will then use the lighter to light one of your wooden kindling sticks. 16. Stick the kindling through the open the door to the large tin can and light your food on fire. You will best be able to start your food on fire if you are able to burn underneath the food, burning the food from bottom up. 17. Once the the food fire is out, you will record the final Temperature of the water and final Mass of the food sample. 18. Repeat Steps 11-17 for how many trials/food items you want to burn. Data : Example: Doritos #2 In this lab we will use the Q= M *C *∆T formula and the percent error formula Mass of water * Specific Heat of Water * Change in Temperature= Joules 200 g * 4.184 J/g *C * 96.4- 23.4= 73*C = 61,086.4 J Change 61,086.4 J to Cal J / (4.184 J * 1000 cal) = Experimental Calories divide by 4.184 and 1000 because there is 1 cal in 4.184 J and 1000 cal in one Cal 61,086.4 J/ (4.184 J Mass Burned * 3.08g * * 1000 cal) = 14.6 Cal Calories/gram = Actual Calories 150/28= 5.36 cal/g =16.5 Cal Percent Error= Actual Calories- Experimental Calories / Actual Calories 11.51% = 16.5 Cal Data Table: - 14.6 Cal / 16.5 Cal Doritos Initial Final Mass Initial Final % Mass Mass Of Temp Temp Burn (g) (g) Water *C *C 4.23 3.83 200 23.6 32.3 9.45 3.98 .9 200 23.4 96.4 .85 .09 150 26.5 2.2 1.35 200 23.2 21.9 2.5 2.59 Cal/g Exp. Actual % Cal Cal Error 5.36 1.74 2.144 18.84 77.38 5.36 14.6 16.5 11.51 45.1 89.41 5.71 2.79 4.34 35.71 22.5 39.2 38.6 5.40 3.34 4.59 27.23 200 24.8 33.6 14.22 4.09 1.76 5.317 63.55 1.6 200 24.6 41.3 36 5.71 3.34 5.139 35.01 2.1 200 24.1 33.4 18.92 5.71 1.86 2.80 33.57 #1 Doritos #2 Planters Peanuts Cheetos Puffs Rice Krispie Treats Lay’s Potato Chips Fritos Conclusion: My hypothesis was incorrect because the order from least percent error to greatest is Doritos #2, Doritos, Cheetos Puffs, Fritos, Lay’s Potato Chips, Planters Peanuts, and Rice Krispie Treats. In the lab, I had two separate trials of Doritos because I had two great burns on my Doritos. Doritos were the most accurate. Doritos had the least amount of percent error, at 18.84% and 11.51%, because the Doritos had the most mass to start with and had the best flame compared to the rest of the food items. The mass would help to create a less percent error because if you are off by tenths of a gram, it’s not tens of degrees off like a peanut but a few degrees if not less. The largest flame would also help because it would heat the water up the best. The Cheetos Puffs was the second most accurate, at 27.23%, because the Cheetos Puffs had the third largest percent burn. More percent burn would help to heat the water up and reduce the percent error. The next three foods all had a similar percent error. In fact there was only two percent separating the Fritos (33.57%) from the Lay’s Potato Chips (35.01%) and the Planters Peanuts (35.71%). The Fritos and the Lay’s Potato Chip had similar percent errors because they both had similar masses and percent burns. They had some of the lowest percent burns, causing the water temps to barely change and their percent error to be high. The Planters Peanuts had the second worst percent because it had the least amount of mass. This made it the food item with the least amount of actual calories, so when it was off by less than two calories, it was a large percent error. When figuring out my lab setup, I tried many different types of setups. At first I only had three holes in the top of my can. This didn’t allow enough of the smoke to get out and allow oxygen to get into my calorimeter, so I made seven holes instead. I also tried many different ways to burn our food. At first I tried to burn different items by lighting them when they were just sitting on the ground. The items barely caught on fire and had a very small percent burn. Then I started propping up the chips on top of other chips. This had a better percent burn but not the percent burn I had wanted. Then, I used a paperclip to hold the Planters Peanuts, and to prop the different chips against. By doing this, the chips had a better percent burn and heated the water up better with the paperclip. Before I began placing foods in the calorimeter, I tried burning foods in the fume hood to see how well they burned. I did this to know how well the food items burned to know what to expect when they were in the calorimeter. Doing this, I knew water-based items like apples would not work in our experiment, but I didn’t realize that gummy bears would not burn. When I lit the gummy bear on fire, it just melted and never truly lit. Also, when we placed the items under the fume hood, I had a pretty good idea that the Doritos and the Planters Peanuts would have a large percent burn. So when I first lit the Doritos in the calorimeter and saw it go out right away, I knew that there wasn’t enough oxygen able to get to the Doritos. So my partner and I decided to raise our large tin can off the ground for the rest of the lab, and had enough oxygen without releasing too much heat. In my lab, my experimental calories were always less than the actual number of calories. This was because I was losing some of the heat given off by the food items. This occurred because not all of the heat released from the food items heated up the water. A little heat escaped through the air holes in the top of the can and on the sides of the calorimeter when I raised it. Some heat was lost because it was heating the air inside of the large can. Also, some of the heat escaped the large tin can because the sides of the can were touching the outside air. Sources ● http://www.symptomfind.com/ ● http://kidshealth.org/ ● http://articles.latimes.com/ ● http://www.todayifoundout.com/