Synthesis and Characterization of Peptide Nucleic Acid for
advertisement
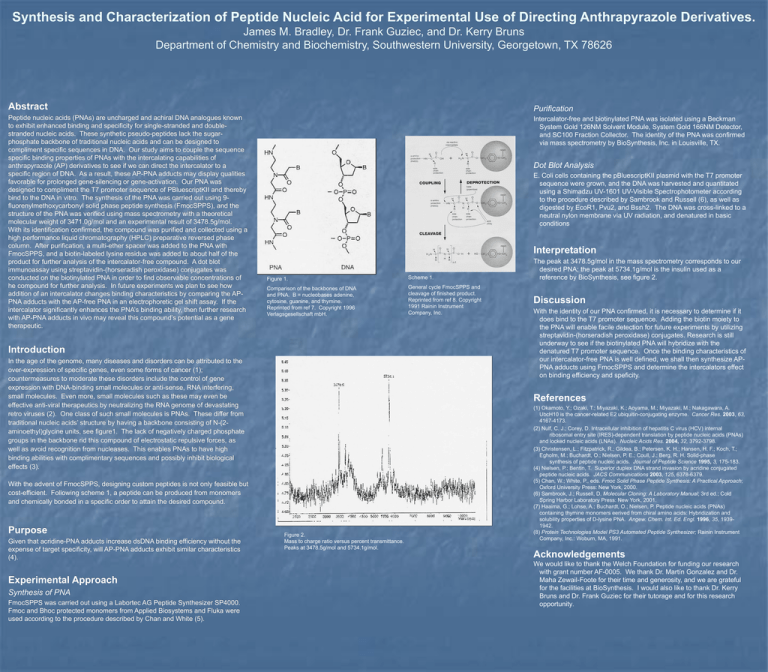
Synthesis and Characterization of Peptide Nucleic Acid for Experimental Use of Directing Anthrapyrazole Derivatives. James M. Bradley, Dr. Frank Guziec, and Dr. Kerry Bruns Department of Chemistry and Biochemistry, Southwestern University, Georgetown, TX 78626 Abstract Purification Peptide nucleic acids (PNAs) are uncharged and achiral DNA analogues known to exhibit enhanced binding and specificity for single-stranded and doublestranded nucleic acids. These synthetic pseudo-peptides lack the sugarphosphate backbone of traditional nucleic acids and can be designed to compliment specific sequences in DNA. Our study aims to couple the sequence specific binding properties of PNAs with the intercalating capabilities of anthrapyrazole (AP) derivatives to see if we can direct the intercalator to a specific region of DNA. As a result, these AP-PNA adducts may display qualities favorable for prolonged gene-silencing or gene-activation. Our PNA was designed to compliment the T7 promoter sequence of PBluescriptKII and thereby bind to the DNA in vitro. The synthesis of the PNA was carried out using 9fluorenylmethoxycarbonyl solid phase peptide synthesis (FmocSPPS), and the structure of the PNA was verified using mass spectrometry with a theoretical molecular weight of 3471.0g/mol and an experimental result of 3478.5g/mol. With its identification confirmed, the compound was purified and collected using a high performance liquid chromatography (HPLC) preparative reversed phase column. After purification, a multi-ether spacer was added to the PNA with FmocSPPS, and a biotin-labeled lysine residue was added to about half of the product for further analysis of the intercalator-free compound. A dot blot immunoassay using streptavidin-(horseradish peroxidase) conjugates was conducted on the biotinylated PNA in order to find observable concentrations of he compound for further analysis. In future experiments we plan to see how addition of an intercalator changes binding characteristics by comparing the APPNA adducts with the AP-free PNA in an electrophoretic gel shift assay. If the intercalator significantly enhances the PNA’s binding ability, then further research with AP-PNA adducts in vivo may reveal this compound’s potential as a gene therapeutic. Intercalator-free and biotinylated PNA was isolated using a Beckman System Gold 126NM Solvent Module, System Gold 166NM Detector, and SC100 Fraction Collector. The identity of the PNA was confirmed via mass spectrometry by BioSynthesis, Inc. in Louisville, TX. Dot Blot Analysis E. Coli cells containing the pBluescriptKII plasmid with the T7 promoter sequence were grown, and the DNA was harvested and quantitated using a Shimadzu UV-1601 UV-Visible Spectrophotometer according to the procedure described by Sambrook and Russell (6), as well as digested by EcoR1, Pvu2, and Bssh2. The DNA was cross-linked to a neutral nylon membrane via UV radiation, and denatured in basic conditions Interpretation Figure 1. Scheme 1. Comparison of the backbones of DNA and PNA. B = nucleobases adenine, cytosine, guanine, and thymine. Reprinted from ref 7. Copyright 1996 Verlagsgesellschaft mbH. General cycle FmocSPPS and cleavage of finished product. Reprinted from ref 8. Copyright 1991 Rainin Instrument Company, Inc. Introduction In the age of the genome, many diseases and disorders can be attributed to the over-expression of specific genes, even some forms of cancer (1); countermeasures to moderate these disorders include the control of gene expression with DNA-binding small molecules or anti-sense, RNA interfering, small molecules. Even more, small molecules such as these may even be effective anti-viral therapeutics by neutralizing the RNA genome of devastating retro viruses (2). One class of such small molecules is PNAs. These differ from traditional nucleic acids’ structure by having a backbone consisting of N-(2aminoethyl)glycine units, see figure1. The lack of negatively charged phosphate groups in the backbone rid this compound of electrostatic repulsive forces, as well as avoid recognition from nucleases. This enables PNAs to have high binding abilities with complimentary sequences and possibly inhibit biological effects (3). Given that acridine-PNA adducts increase dsDNA binding efficiency without the expense of target specificity, will AP-PNA adducts exhibit similar characteristics (4). Experimental Approach Synthesis of PNA FmocSPPS was carried out using a Labortec AG Peptide Synthesizer SP4000. Fmoc and Bhoc protected monomers from Applied Biosystems and Fluka were used according to the procedure described by Chan and White (5). Discussion With the identity of our PNA confirmed, it is necessary to determine if it does bind to the T7 promoter sequence. Adding the biotin moiety to the PNA will enable facile detection for future experiments by utilizing streptavidin-(horseradish peroxidase) conjugates. Research is still underway to see if the biotinylated PNA will hybridize with the denatured T7 promoter sequence. Once the binding characteristics of our intercalator-free PNA is well defined, we shall then synthesize APPNA adducts using FmocSPPS and determine the intercalators effect on binding efficiency and speficity. References With the advent of FmocSPPS, designing custom peptides is not only feasible but cost-efficient. Following scheme 1, a peptide can be produced from monomers and chemically bonded in a specific order to attain the desired compound. Purpose The peak at 3478.5g/mol in the mass spectrometry corresponds to our desired PNA; the peak at 5734.1g/mol is the insulin used as a reference by BioSynthesis, see figure 2. Figure 2. Mass to charge ratio versus percent transmittance. Peaks at 3478.5g/mol and 5734.1g/mol. (1) Okamoto, Y.; Ozaki, T.; Miyazaki, K.; Aoyama, M.; Miyazaki, M.; Nakagawara, A. UbcH10 is the cancer-related E2 ubiquitin-conjugating enzyme. Cancer Res. 2003, 63, 4167-4173. (2) Nulf, C. J.; Corey, D. Intracellular inhibition of hepatitis C virus (HCV) internal ribosomal entry site (IRES)-dependent translation by peptide nucleic acids (PNAs) and locked nucleic acids (LNAs). Nucleic Acids Res. 2004, 32, 3792-3798. (3) Christensen, L.; Fitzpatrick, R.; Gildea, B.; Petersen, K. H.; Hansen, H. F.; Koch, T.; Egholm, M.; Buchardt, O.; Nielsen, P. E.; Coull, J.; Berg, R. H. Solid-phase synthesis of peptide nucleic acids. Journal of Peptide Science 1995, 3, 175-183. (4) Nielsen, P.; Bentin, T. Superior duplex DNA strand invasion by acridine conjugated peptide nucleic acids. JACS Communications 2003, 125, 6378-6379. (5) Chan, W.; White, P., eds. Fmoc Solid Phase Peptide Synthesis: A Practical Approach; Oxford University Press: New York, 2000. (6) Sambrook, J.; Russell, D. Molecular Cloning: A Laboratory Manual; 3rd ed.; Cold Spring Harbor Laboratory Press: New York, 2001. (7) Haaima, G.; Lohse, A.; Buchardt, O.; Nielsen, P. Peptide nucleic acids (PNAs) containing thymine monomers eerived from chiral amino acids: Hybridization and solubility properties of D-lysine PNA. Angew. Chem. Int. Ed. Engl. 1996, 35, 19391942. (8) Protein Technologies Model PS3 Automated Peptide Synthesizer; Rainin Instrument Company, Inc.: Woburn, MA, 1991. Acknowledgements We would like to thank the Welch Foundation for funding our research with grant number AF-0005. We thank Dr. Martín Gonzalez and Dr. Maha Zewail-Foote for their time and generosity, and we are grateful for the facilities at BioSynthesis. I would also like to thank Dr. Kerry Bruns and Dr. Frank Guziec for their tutorage and for this research opportunity.