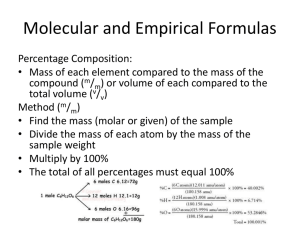
Empirical & Molecular Formula Practice
advertisement

Name: ___________________________________________________ Date: _________________ Period: __________ Empirical & Molecular Formula Practice 1. Vitamin C is composed of C, H, and O. When a 1.00 g sample was analyzed it was found to contain the amounts of C, H, and O as indicated in the table below. Use the information to find the empirical formula of Vitamin C. Element Mass if 1.00 g of Vitamin C C 0.409 g H 0.046 g O 0.545 g 2. A compound composed of calcium and bromine have the respective percentages of 20.0% and 80.0%. Determine the empirical formula of this compound. 3. Find the empirical formula for a compound that contains 36.5% sodium, 25.4% sulfur, and 38.1% oxygen. 4. What is the empirical formula for a compound which contains 53.73% iron and 46.27% sulfur? 5. What is the empirical formula of a compound composed of 15.77% Al, 28.11% S, and 56.12% O? 6. The empirical formula of a compound is NO2. Its molecular mass is 92 g/mol. What is the molecular formula? 7. The empirical formula of a compound is CH2. Its molecular mass is 70 g/mol. What is its molecular formula? 8. A compound is found to be 40% carbon, 6.7% hydrogen, and 53.5% oxygen. Its molecular mass is 60. g/mol. What is its molecular formula? 9. A compound is 64.9% carbon, 13.5% hydrogen, and 21.6% oxygen. Its molecular mass is 74 g/mol. What is its molecular formula? 10. para-1.4-benzenedicarboxylic acid is 57.83% carbon by mass, 38.52% oxygen by mass and 3.65% hydrogen by mass. If the molar mass of the compound is 166.14 g/mol, what is the molecular formula of the compound?