Chapter10
advertisement

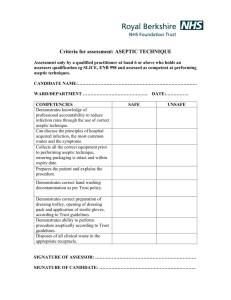
Chapter 10 Infection Control and Safe Handling of Hazardous Agents Learning Objectives • Explain the germ theory of disease—the role of pathogenic organisms in causing disease. • Distinguish among viruses, bacteria, fungi, and protozoa. • Discuss the advantages and disadvantages of various forms of sterilization. • Identify sources and prevention of common causes of contamination. Learning Objectives • Describe proper aseptic technique, including the use of horizontal and vertical laminar airflow hoods and the new United States Pharmacopeia requirements. • Discuss the importance of and techniques for handling and disposing of hazardous agents. INFECTION CONTROL • Development of the Germ Theory of Disease: The identification of microorganisms as a cause of infectious disease is a surprisingly recent development. Pasteur was the originator of the theory. In one experiment, he successfully immunized chickens against chicken cholera. INFECTION CONTROL • Micoorganisms and Disease: Since the days of Pasteur, thousands of pathogenic, or diseasecausing, microorganisms have been identified. Bacteria, fungi, viruses, and protozoa are examples of microorganisms that can be harmful. INFECTION CONTROL • Asepsis and Sterilization: The preparation of parenteral products, especially those for IV administration, requires special aseptic techniques, equipment, and procedures to minimize contamination. Various types of sterilization are available to kill microorganisms from medical instruments, devices, and surfaces. Figure 10.1 INFECTION CONTROL • Contamination: Accidental exposures require immediate treatment, cleanup, reporting to the supervisor, and completion of an incident report. Contanination can occur in a pharmacy by three primary means: touch, air and water. Terms to Remember • • • • • • • spontaneous generation germ theory of disease viruses bacteria fungi protozoa asepsis Terms to Remember • sterilization • autoclave ASPTIC TECHNIQUE AND USP CHAPTER 797 Aseptic technique is used for the preparation of parenteral admixtures, combinations of fluids and/or medications or nutrients, which are administered using bolus (i.e., push) or other intravenous (IV) methods. Table 10.1 summarizes the steps in detail. Aseptic Technique 1. Remove all jewelry (e.g., watches, rings, bracelets, necklaces. Table 10.1 Aseptic Technique 2. Put on non-shedding coats, gowns, or coveralls (hospital scrubs); head and facial hair covers; face masks; and shoe covers. Note that it is important to follow the sequence of items indicated in this step. Table 10.1 Aseptic Technique 3. Scrub hands and arms to the elbows thoroughly with an antiseptic cleanser (e.g., povidone-iodine or chlorhexidine gluconate). Table 10.1 Aseptic Technique 4. Clean the laminar flow hood with isopropyl alcohol. The alcohol must remain in contact with the surface for 30 seconds prior to compounding any sterile product. Table 10.1 Aseptic Technique 5. Place only essential materials under the airflow hood—no paper, pens, or labels. Remove the selected syringe(s) from its overwrap, attach a needle, then discard the waste. Table 10.1 Aseptic Technique 6. Scrub again and glove. Table 10.1 Aseptic Technique 7. Swab or spray needle-penetration closures on vials, injection ports, and other materials. Table 10.1 Aseptic Technique 8. Prepare the sterile product by withdrawing the medication from vials or ampules and introducing it into the IV container. Table 10.1 Aseptic Technique 9. Complete a quality check of the product for container integrity and leaks, solution cloudiness, particulates, color of solution, and proper preparation of product. Table 10.1 Aseptic Technique 10. Present the product, the containers and devices used, and the label to a pharmacist for verification of the product preparation. Table 10.1 Handling Issues Safety Note! • When working in the pharmacy lab clean room, it is important to wear appropriate sterile gear, including face mask, full head covering, scrubs or gown with back closure, and gloves. Figure 10.2 Figure 10.3 Handling Issues Safety Note! • Coughing and talking should be directed away from the hood. Terms to Remember • aseptic technique • horizontal laminar airflow hood • high-efficiency particulate air (HEPA) filter • vertical laminar airflow hood • quality assurance (QA) program • USP Chapter 797 Terms to Remember • compounded sterile products (CSPs) • beyond-use dating HANDLING AND DISPOSAL OF HAZARDOUS AGENTS • Receipt and Storage of Hazardous Agents: Hazardous drugs should be delivered directly to the storage area, inventoried, and, if necessary, refrigerated. Access to storage areas and work areas for hazardous materials should be limited to specified trained personnel. HANDLING AND DISPOSAL OF HAZARDOUS AGENTS • Protective Clothing: A disposable, lintfree, nonabsorbent, closed-front gown with cuffed sleeves should be worn. Hair and shoe covers should be worn to reduce the potential for particulate contamination. Other protective clothing would include eye protection, mask, and use of latex gloves when disposing of damaged packages. HANDLING AND DISPOSAL OF HAZARDOUS AGENTS • Technique for Handling Hazardous Agents: Hazardous drugs such as cancer chemotherapy require special techniques, equipment, and procedures to protect the health of the employee. • Hazardous Agent Spills: Accidental exposures require immediate treatment, cleanup, reporting to the supervisor, and completion of an incident report. HANDLING AND DISPOSAL OF HAZARDOUS AGENTS • Procedures in Case of Exposure: Any body area exposed should be flooded with water and thoroughly cleansed with soap and water. Dispose of contaminated garments appropriately in specially designated biohazard materials containers. HANDLING AND DISPOSAL OF HAZARDOUS AGENTS • Hazardous Oral Dose Forms: The counting and pouring of these drugs should be done carefully, and contaminated equipment such as counting trays should be immediately cleaned with detergent and rinsed. Compounding with any of these drugs should be done in a protected area away from drafts and traffic. Handling Issues Safety Note! • With hazardous drugs, inject a volume of air that is no more than 75% of the amount of drug to be withdrawn. Terms to Remember • cytotoxic materials • antineoplastic drugs Discussion Select and research a drug from the hazardous drug list (Table 10.2) and provide a written or verbal report on its potential toxicity to a pharmacy technician exposed to it. Is toxicity the result of skin irritation, ingestion, or inhalation? Discussion The JCAHO is due to visit your hospital for accreditation. Your hospital’s ICC is asking your department of pharmacy to develop quality assurance (QA) policy on your IV admixture service. Your pharmacist supervisor has designated you to write the first draft. Check the following Web site for information: www.ashp.org/bestpractices/drugdistribution/Pr ep_Gdl_QualAssurSterile.pdf