Unit 31
advertisement

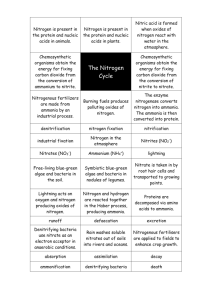
Unit 31 Nitrogenous fertilizers & sulphuric acid How do green plants make their own food? By Photosynthesis Green plants make use of carbon dioxide and water, in the presence of light and chlorophyll, make glucose (starch) and oxygen. What are needed for healthy plant? Green plants make their food in photosynthesis. Any more things needed? Mineral salts (elements) are needed. What are they? They are nitrogen, phosphorus, and potassium. These are removed from the soil when crops are harvested. Thus, fertilizers have to be added for restoring minerals to the soil. Why these elements are good for plants? Types of fertilizers Natural fertilizers - compost (decaying organic matter) and manure. Artificial fertilizers - a mixture of compounds containing the elements, nitrogen, phosphorus and potassium. (e.g., ammonium sulphate or amonium nitrate) Changing nitrogen gas (in air) into compounds How? In thunderstorm (lightning) (with electric spark), nitrogen and oxygen combine to form nitrogen oxide. N2(g) + O2(g) 2NO(g) Nitrogen monoxide then combines with oxygen in air to form nitrogen dioxide. 2NO(g) + O2(g) 2NO2(g) Changing nitrogen gas into compounds Nitrogen dioxide dissolves in rainwater to form dilute nitric acid. 2NO2(g) + H2O(l) HNO2(aq) + HNO3(aq) 4NO2(g) + O2(g) + 2H2O(l) 4HNO3(aq) Sources of nitrate ions (natural fertilizers). Nitrogen in soil Nitrogen-fixing bacteria in the roots of peas and beans for changing nitrogen into nitrates. Nitrogenous fertilizers Nitrogenous compound Physical state Molar mass (g mol-1) solubility gas 17 very high ammonium nitrate, NH4NO3 solid 80 extremely high ammonium sulphate, (NH4)2SO4 solid 132 high ammonium hydrogenphosphate, (NH4)2HPO4 solid 132 low Urea, (NH2)2CO solid 60 high ammonia, NH3 Percentage by mass of nitrogen in fertilizers Calculate the percentage by mass of nitrogen in ammonia nitrate. Formula of ammonium nitrate ? Mass of 1 mole of ammonium nitrate ? Mass of nitrogen in 1 mole of ammonium nitrate ? Mass percentage of nitrogen in ammonium nitrate ?? Percentage by mass of nitrogen in ammonium sulphate Formula of ammonium sulphate ? Molar mass of ammonium sulphate ? Mass percentage of nitrogen in ammonium sulphate ?? Calculation example 1 kg of ammonium sulphate is sold at a price of $2. Calculate the cost of buying 1 kg of nitrogen by buying ammonium sulphate. NPK label Nitrogen Number The mass percentage of nitrogen in the fertilizer. If the nitrogen number of a fertilizer is 15, Calculate the mass of nitrogen in 1000 g fertilizer. Phosphorus Number the mass percentage of phosphorus in the form of P2O5 present in the fertilizer. If the NPK label of a fertilizer is 16.8.24., calculate the mass percentage of phosphorus in the fertilizer. Potassium number the mass percentage of potassium in the form of K2O present in the fertilizer. If the NPK label of a fertilizer is 16.8.24., calculate the mass percentage of potassium in the fertilizer How to prepare a fertilizer (ammonium sulphate) in the laboratory? How?? What chemicals are needed? What are their formulae? Write an equation for the reaction involved. Experimental Steps (Procedures) Pipette 25.0 cm3 ammonia solution into a conical flask. Add a few drops of methyl orange (indicator). Colour in alkali: yellow Fill the burette with dilute sulphuric acid. Titrate the ammonia solution with dilute sulphuric acid until the methyl indicator turns from ______ to ______ . (I.e. the end point is reached.) Experimental Steps (Procedures) Mix 25.0 cm3 of ammonia solution with the required volume of dilute sulphuric acid without any indicator. Warm to saturate the salt solution. Allow it to crystallize slowly to give large crystals of ammonium sulphate. Filter off the crystals and dry crystals between filter paper. Haber Process Industrial Manufacture of ammonia By the direct combination of nitrogen and hydrogen In the presence of fine divided iron catalyst. N2(g) + 3H2(g) 2NH3(g) What is a catalyst? Name an enzyme (a biological catalyst). A catalyst is a substance that will alter / change (usually speed up) the rate of a chemical reaction. Catalysts are usually transition metals or compounds of transition metals. Flow diagram of Haber Process Raw materials of Haber Process Nitrogen – from the fractional distillation of liquid air Hydrogen – from the reaction of steam with methane or naphtha. CH4(g) + H2O(g) CO(g) + 3H2(g) Finely divided iron catalyst Fine-divided – in powder form Why ? To Increase the surface area of the catalyst. ‘Poisoning’ of the catalyst Impurities, such as sulphides and carbon monoxide, adhere on the surface of the solid catalyst. – “poisoning of the catalyst”. Raw materials, nitrogen and hydrogen are purified, before passing over the redhot catalyst. Reaction Conditions of Haber Process Nitrogen to hydrogen volume ratio =1:3 Compressed to a pressure of 200 atmospheres. Pass over the red-hot iron catalyst at 500oC. N2(g) + 3H2(g) 2NH3(g) Exothermic Percentage yield : 15% Ways to improve (increase) the yield of ammonia By removing ammonia (product) from the reaction mixture. By liquefying ammonia or by dissolving ammonia in water. Removing ammonia and the recycle of unreacted nitrogen and hydrogen. Increase the yield of ammonia. (More ammonia) Heat released in the reaction is absorbed by the heat exchanger for heating the incoming new reactants. Preparing ammonia in the laboratory Ammonia – a weak alkali. Prepared by heating a salt of weak alkali with a strong alkali. Heating ammonium salt with a strong alkali. NH4Cl + NaOH NH3 + NaCl + H2O A colourless gas with irritating smell which turns moist (wet) red litmus paper blue is given out. Ammonia gas is poisonous. Heating ammonium salt with sodium hydroxide Test for ammonia gas With a characteristic irritating smell (no a chemical test) Turns moist red litmus paper blue. NH3(aq) + H2O(l) NH4+(aq) + OH-(aq) Put a glass rod wetted with concentrated hydrochloric acid near a gas jar (tube) of ammonia gas. A dense white fume of ammonium chloride is formed. NH3(g) + HCl(g) NH4Cl(s) Ammonia solution as a weak alkali What is a weak alkali? Partly ionized in aqueous solution. NH3(aq) + H2O(l) NH4(aq) + OH-(aq) What do you see when limited amount of aqueous ammonia is added into copper(II) sulphate solution? Pale blue precipitate is formed. Cu2+(aq) + 2OH-(aq) Cu(OH)2(s) Uses of ammonia gas To fix atmospheric nitrogen into nitrogen compounds which are used as fertilizers. A good solvent for grease. Used as a glass (window) cleaner. Uses of ammonia gas Used as a freezing agent (refrigerant) in refrigerators. An important starting chemical for preparing nitric acid and fertilizers. Industrial manufacture of nitric acid (Ostwald Process) Oxidation number of nitrogen in ammonia ?? Oxidation number of nitrogen in nitric acid ??? By catalytic oxidation of ammonia Ammonia is oxidized catalytically by oxygen in the presence finely divided platinum catalyst. 4NH3(g) + 5O2(g) 4NO(g) + 6H2O(g) Industrial manufacture of nitric acid (Ostwald Process) Nitrogen monoxide reacts with oxygen (in air) to form nitrogen dioxide. 2NO(g) + O2(g) 2NO2(g) Nitrogen dioxide is then dissolved in water in the presence of oxygen to form nitric acid 4NO2(g) + O2(g) + 2H2O(l) 4HNO3(aq) Uses of nitric acid For making fertilizers. For making monomers of the polymer – nylon. For making explosives. For making drugs. Contact Process for the manufacture of concentrated sulphuric acid Raw materials: ??? Sulphur dioxide, SO2 By burning sulphur in air S(s) + O2(g) SO2(g) By roasting metal sulphide ore 2ZnS(s) + 3O2(g) 2ZnO(s) + 2SO2(g) Oxygen from air Contact Process To prevent the ‘poisoning’ of the catalyst by purifying the raw materials. Catalyst used Finely divided platinum – expensive, (more efficient) Or vanadium(V) oxide, V2O5 (cheaper) Reaction conditions Sulphur dioxide and oxygen mixed in the ratio of 2 to 1. Temperature : 450oC A pressure of one or two atmospheres. 2SO2(g) + O2(g) 2SO3(g) Exothermic Percentage yield of SO3 : 90% Flow diagram of contact process Increasing the yield of sulphur trioxide By removing sulphur trioxide. Not by dissolving sulphur trioxide in water as the reaction is highly exothermic and vaporize the acid. The acid fume is hazardous to the operator and harmful to the machinery. Removing sulphur trioxide Dissolving sulphur trioxide in concentrated sulphuric acid to form oleum (fuming sulphuric acid) SO3(g) + H2SO4(l) H2S2O7(l) Oleum is then carefully diluted in right proportion to give concentrated sulphuric acid H2S2O7(l) + H2O(l) 2H2SO4(l) Uses of sulphuric acid For the manufacturing of fertilizers. For the manufacture of soapless detergents and dyestuffs.. Paint Additives For the manufacture of paint additives Metal sulphates are soluble in water except calcium sulphate, barium sulphate and lead(II) sulphate Mixing a soluble calcium / barium salt solution with a soluble sulphate solution. Ca2+(aq) + SO42-(aq) CaSO4(s) Ba2+(aq) + SO42-(aq) BaSO4(s) Uses of concentrated sulphuric acid