Determining the Molecular Mass by Freezing Point Depression
advertisement

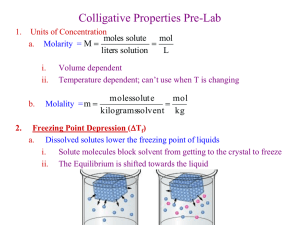
Determining the Molecular Mass by Freezing Point Depression By: Justin Green Freezing Point Depression Freezing point depression is the decrease of the freezing temperature of a solvent after a solute has been added www.chemprofessor.com This shows the freezing point of GEOMELT before and after adding a solute. The solute lowers the freezing point. Freezing Point Depression Ex. The freezing point of salt water is lower than that of pure water http://www.princeton.edu/~pccm/outreach/scsp/water_on_e arth/salt/thermoms.gif Molar Mass The mass of one mole (6.022 x 1023 molecules) of any substance: • Is given on the Periodic Table of Elements Formulas Molar Mass can be found through the formulas following with Freezing Point Depression: M = wKf w= mass fraction of solute ΔT Kf = cyroscopic constant ΔT = change in temperature ΔTf = iKfm I = van’t Hoff value Kf = molal freezing point constant m = concentration in molality Formulas to use ΔTf = I x kf x moles of solute kg of solvent ΔTf = I x kf x m ΔTf = (g solute) (MMsolute) kg solvent MMsolute = I x kf x g solute kg of solvent x ΔTf Purpose To understand how freezing point depression and molecular mass correlate To be able to use new knowledge of freezing point depression in a real world problem Materials required Medium sized test tubes 4.0g Naphthalene 52.0g paradichlorobenzene Thermometer Hot Water Bath (250 mL Beaker and 100 mL water on Hot Plate) Procedures: Determining freezing point 1. Weigh out 4g of naphthalene 2. Weigh out 52g of paradichlorobenzene Procedures 3. Place test tube containing chemicals in hot water bath 4. Place both substances in the hot water bath and allow them to completely melt and stir well http://www3.moe.edu.sg/edumall/tl/digital_resources/biology/images/hot_water_bath.jpg Procedures 5. Remove from the hot water bath and allow it to cool while stirring gently to prevent super cooling http://cn1.kaboodle.com/hi/img/2/0/0/3e/c/AAAAAs-rj2IAAAAAAD7AHg.jpg Procedures 6. Take the temperature (down to 0.1 degrees Celsius) of the solvent every 60 seconds until the solution is solidified http://wolfesscience.com/images/lab-thermometer.jpg Freezing Point Graph I drew this one myself Analysis Questions 1. What is the freezing point of the final solution? 48.4 °C 2. What was the freezing point depression of the solution? The standard freezing point for paradichlorobenzene is 53.0 degrees Celsius ΔTf= T°f – Tf = 53.0°C – 48.4°C = 4.6°C Analysis Questions 3. What was the molality of the naphthalene? The freezing point depression constant for paradichlorobenzene is 7.1°C x m-1 m = ΔTf = 4.6°C = 0.65m kf 7.1°C x m-1 Analysis Questions 4. What was the molecular mass of naphthalene? MMsolute= kf x g solute = 7.1°C x kg kg solvent x ΔTf mol x .052 kg x 4.6°C 120 g mol-1 Analysis Questions 5. What was the percent error of the results compared to the actual molecular mass of 128 g x mol-1 120 – 128 x 100% = -6% 128 The results were 6% below expected Conclusion In conclusion, the molecular mass of an unknown solute can be determined through freezing point depression Freezing Point Depression By: Justin Green