Ch. 15 Sections 15.6
advertisement

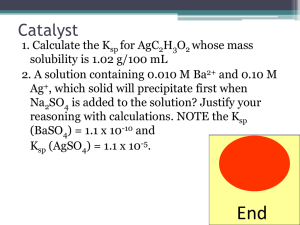
•Solubility is a very important phenomenon. •We can flavor foods due to the solubility of salt and sugar in water. •In this section, we will consider sparingly soluble substances and quantitative ways to determine “How soluble?”. •According to the solubility rules previously learned, PbCl2 and AgCl are both insoluble salts. •However, if chloride ion is added to a solution containing both Pb2+ and Ag+ ions, nearly all of the Ag+ is precipitated as AgCl before any Pb2+ separates from the solution as PbCl2. •This occurs because AgCl is much less soluble than PbCl2. •To explain these differences in solubility, solubility equilibrium must be examined quantitatively. •When placed in water, a small amount of AgCl dissolves and the following equilibrium is established once the solution becomes saturated: AgCl(s) ⇌Ag+(aq) + Cl-(aq) Equilibrium expression: If reactants or products have a coefficient other than + Ksp = [Ag ][Cl ] 1 concentrations must be raised to that power. Ksp = solubility product constant Ksp equals the product of concentration terms for the ions dissolved in a saturated solution of a sparingly soluble substance. •The solubilities of salts change with temperature so a value of Ksp applies only to solutions only at the temperature at which its value was determined. • Ksp can be obtained from a salt’s molar solubility in water – the number of moles of solute dissolved in one liter of its saturated solution. • Examples • Molar solubility can also be computed (estimated) from values of Ksp. • Examples •Suppose we stir some calcium carbonate in water long enough to establish the following equilibrium: CaCO3 (s) ⇌ Ca2+ (aq) + CO32- (aq) •Then we add to the solution a very soluble salt of calcium, like CaCl2. •This puts Ca2+ into solution, and it upsets the above equilibrium. •The ion product is no longer equal to Ksp. •Remember from Le Chatelier’s principle, if we add then equilibrium shifts opposite. •The above equilibrium shifts to the left causing CO32to precipitate as CaCO3. •Eventually equilibrium is reestablished, but with a lower concentration of CO32+ in solution. •In this new system, there are two sources of Ca2+, the added CaCl2 and the CaCO3 still in solution. •Because Ca2+ is common to both sources, it is called a common ion. •The addition of the common ion lowers the solubility of CaCO3; it is less soluble in the presence of CaCl2 (or any other soluble calcium salt) than it is in pure water. •This lowering of the solubility of an ionic compound by the addition of a common ion is called the common ion effect. •The common ion effect can dramatically lower the solubility of a salt. •So far we have considered solids dissolving in solutions. •Now we will consider the reverse process – the formation of a solid from a solution. •We will use the ion product. •Ion product (Q) is defined like expression for Ksp but uses initial concentrations instead of equilibrium concentrations. •Precipitate will form •No precipitate will form •No precipitate will form Ion product >Ksp (supersaturated) Ion product = Ksp (saturated) Ion product < Ksp (unsaturated) •The pH of a solution can affect a salt’s solubility. •Example: Mg(OH)2(s) ⇌ Mg2+(aq) + 2OH-(aq) Add OH(increase basicity) Decrease Solubility of Mg(OH)2 Add H+ (increase acidity -removes OH- by reaction to produce H2O) Increases solubility (lose OHand must replace) •General rule – If the anion X- is an effective base (HX is a weak acid) the salt MX will show increased solubility in an acidic solution. •A complex ion is a charged species consisting of a metal ion surrounded by ligands. •A ligand is simply a Lewis base. •Recall a Lewis base is an ion or molecule having a lone electron pair that can be donated to an empty orbital on the metal ion to form a covalent bond. •Some common ligands are H2O, NH3, Cl-, and CN-. •Metal ions add ligands one at a time in steps characterized by equilibrium constants called formation constants or stability constants. •For example, when solutions containing Ag+ and NH3 molecules are mixed, the following reactions take place: Ag+ (aq) + NH3 (aq) ⇌ Ag(NH3)+ (aq) K1 = 2.1 x 103 Ag(NH3)+(aq) + NH3(aq)⇌ Ag(NH3)2+(aq)K2 = 8.2 x 103 where K1 and K2 are the formation constants for the two steps. •In a solution containing Ag+ and NH3, all the species NH3, Ag+, Ag(NH3)+, and Ag(NH3)+ exist at equilibrium. •When we write the formula for a complex, we follow two rules: 1. The symbol for the metal ion is always given first, followed by the ligands. 2. The charge on the complex is the algebraic sum of the charge on the metal ion and the charges on the ligands. • • For example, the formula of the complex ion of Cu2+ and H2O is written Cu(H2O)42+ with the Cu first followed by the ligands. The charge on the complex is 2+ because the copper ion has a charge of 2+ and the water molecules are neutral. •Metal ions that commonly form complex ions (or coordination compounds): Al3+, Cu2+, Zn2+, Fe2+ (or 3+), Ni2+, Ag+. (All Curiously Colored Zebras Felt Nicely Agreeable) •Common ligands: NH3, OH-, Cl-, SCN-, CN-, H2O. •Most common coordination number: twice the charge of the metal ion. •That means that Ag+ and NH3 → Ag(NH3)2+. •Formation of complex ions is a common means to dissolve otherwise insoluble salts. •For example, in a solution with only water present, AgCl only dissociates (dissolves) slightly to form Ag+. •When ammonia is added, the Ag+ complexes with the ammonia, and the removal of the Ag+ from the solution as it converts to Ag(NH3)2+ pulls the AgCl dissociation equilibrium to the right (LeChatelier’s Principle). •If sufficient ammonia is added to complex all of the silver ions, the AgCl will completely dissolve. AgCl(s) ⇌ Ag+ (aq) + Cl- (aq)