Acids/Bases Review
advertisement

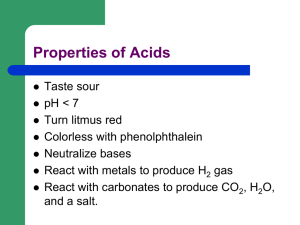
Name: __________________________________________________ Date: ________ ACID BASE EXAM REVIEW Sort the following properties as acids or bases Tastes sour turns litmus blue. Feels slippery KOH releases OH- ions when dissolved in water. releases H+ ions when dissolved in water. H3PO4 found in citrus fruit. Antacids reacts with metals to produce H2 gas. Ammonia [OH-] > [H+] Soap turns bromethyl blue yellow. Vinegar turns litmus paper red. pH < 7 pH > 7 [H+] > [OH-] tastes bitter. 1. Acetic acid is also known as__________ 2. Are acids electrolytes? 3. Are bases electrolytes? 4. The pH scale that is generally used ranges from the numbers _____________. 5. What is the pH of a neutral solution at 25ºC? 6. An aqueous solution whose pH is 4 is a(n)__________. (acid or base) 7. Define “binary acid”. 8. Define “oxyacid” 9. When an acid mixes with a base, they _________________ each other. 10. A Bronstedt-Lowry acid is a _______________. Name: __________________________________________________ Date: ________ 11. A Bronstedt-Lowry base is a ___________________. 12. An Arrhenius acid produces ____________________ in solution. 13. An Arrhenius base produces ____________________ in solution. 14. What theory of acids and bases do conjugate acids and bases belong to? 15. In the reaction HF + H2O H3O+ + F–, the acid-conjugate base pair is 16. In the reaction HClO3 + H2O H3O+ + ClO3–, the conjugate base of HClO3 is 17. Traditionally, an acid is a compound that contains ___________ and will form ____________ in water. 18. The product of H+ and OH– concentrations in water are equal to what constant number? 19. Strong acids are _____________ electrolytes, and vice versa. 20. A polyprotic acid contains ____________ hydrogen atoms. 21. List an example of a weak base. 22. List examples of strong bases. 23. List examples of strong acids. 24. List examples of weak acids. 25. For this reaction, diagram the acid, base, conjugate base, and conjugate acid: NH3 + H2O NH4+ + OH– 26. Use the above reaction to answer the following question: In comparing a conjugate acid and a base, the conjugate acid typically has _________ number of hydrogen than the base. 27. If [H+] of a solution is greater than [OH–], the solution is a _____. 28. The name of a binary acid always ends in __________. 29. The name of a binary acid always starts with the prefix ____________. 30. Write the formula for acetic acid? 31. An oxyacid that has the suffix -ic forms from a polyatomic ion with the ending ___________. Name: __________________________________________________ Date: ________ 32. An oxyacid that has the suffix -ous forms from a polyatomic ion with the ending ___________. 33. What of the formula for chloric acid? 34. What is the formula for chlorous acid? 35. Acidic pH ranges from __________. 36. Basic pH ranges from ___________. 37. How many acid-base pairs participate in a Brønsted-Lowry acid-base reaction? 38. If [H+] = 2.0 10–8 M, what is the pH of the solution? 39. What is the pOH of a solution whose hydrogen ion concentration is 2.6 10–2 M? 40. What is the hydrogen ion concentration of a solution whose pH is 2? 41. What is the pOH of a solution whose pH is 8? 42. What is the hydroxide ion concentration of a solution whose pH is 6.2? 43. What is the pH of a 4.8 x 10–3 M KOH solution? Name: __________________________________________________ Date: ________