H 3 O +
advertisement

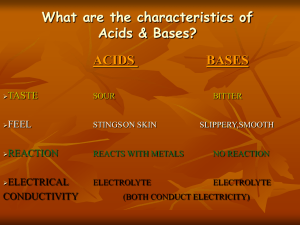
Chapter 14: Acids and Bases and pH Acids 1) Sour taste 2) Litmus paper (common indicator) changes from blue to red in an acid or red litmus paper stays red. 3) Acids always contain hydrogen. • Acids are proton donors (give a hydrogen away) • Bronsted-Lowry definition of an acid 4) Acids react with metals to form hydrogen gas and a metal compound called corrosion Acids – produce hydronium ions in water. HCl(g) + H2O (l) ----- H3O+ (aq) + Cl- (aq) Also known as Arrhenius Acids When a hydrogen atom is attached to a water molecule. Hydronium ion: H3O+ ion is positively charged. Conduct an electrical current (charged particles). Hydrogen is always written first in an acid. •If there is Only one hydrogen – HCl – monoprotic •Diprotic (2) and polyprotic (more than 2) Example: H2SO4 – sulfuric acid - diprotic BUT not all hydrogen containing compounds are acids. Example: sugar C11H22 O11 Note: if hydrogen is not written first – not an acid! Bases 1) taste bitter, feel slippery 2) turn litmus paper from red to blue or blue paper stays blue. 3) emulsify (dissolve) fats and oils 4) Arrhenius bases produce hydroxide ion (OH-) when dissolved in water. (need to know name and symbol) 5) Bases are called proton acceptors Proton acceptors- Bronsted – Lowry definition of a base Common Base - Sodium Hydroxide (NaOH) also known as ‘lye’ common base – very strong. NaOH (s) ------- Na+ (aq) + H2O OH- (aq) OH- hydroxide ion •Example: NH3 (g) + H2O (l) --- NH4+ (aq) + OH- (aq) Ammonia – covalent base •Water does play a role in this reaction •Since NH3 accepts a proton (H+) and is now NH4+ it is considered a base. 14.2 Strengths of Acids and Bases Strong Acids and Bases Completely ionize in water Common acids – know these Strong: •H2SO4 – sulfuric acid •HNO3 – nitric acid •HCl – hydrochloric acid Strong acids : •ionize well in water (lose hydrogen quickly) = good electrolytes Weak acids: HC2H3O2 – acetic acid (acid in vinegar) know this one H2CO3 – carbonic acid (used for carbonating soda) H3PO4 – phosphoric acid (a flavoring agent in soda) H3BO3 – boric acid (eye wash) Weak acids do not ionize well in solution (produce only a few H+ in solution) Poor Electrolytes! Acetic acid Common Bases – •Strong bases ionizes completely in water, produce large # of ions therefore Good Electrolytes. Strong Bases: •KOH – battery electrolyte •NaOH – used for making soap, Drano; know this one •Ca(OH)2 – leather production, making plaster •Mg(OH)2 – laxative, antacid Weak Bases: do not produce large amount of ions, weak electrolytes. •NH4OH – ammonium hydroxide (household cleaner) •Al(OH)3 – aluminum hydroxide (antacid, deodorant) •NH3 Ammonia – household cleaning products know this one •pH scale is used to measure the acidity of solution. Definition: •The pH (‘potential of hydrogen’) measure of hydronium ion (H3O+)concentration. is a •It is a Mathematical scale 0-14. •Logarithmic Scale – based on powers of 10. Example: pH of 3 is 10 times more acidic than a pH 4. pH of 3 is 100 (10 x 10) times more acidic than the pH of 5. pH less than 7 = acidic pH above 7 = basic 7 = neutral (distilled water) To calculate pH scale = take the negative of the exponent of the hydronium ion concentration = -log [H3O+] Concentrations of hydronium and hydroxide in water: [H3O+] x [OH-] = 1 x 10 -14 Example: If concentration of the hydronium ion [H3O+] is 10-8 M. Then the solution has a pH of 8. Example: If the hydroxide ion [OH-] concentration is 10-5 M, then the pH = 10 -14 /10 -5 = 10 -9 -- pH of 9. pH of common materials showing the relationship between pH and the hydronium ion concentration. Neutral Solution: [H3O+] = [OH-] Acid Solution: [H3O+] > [OH-] 15.1 Acid and Base Reactions •The reaction between an acid and a base is called a Neutralization Reaction. •Usually neutralization reactions produce water and a salt. •NaOH (aq) + HCl (aq) ----- NaCl (aq) + H2O (l) •pH = 7 •Salt – Ionic compound formed from the negative part of the acid and the positive part of the base. Neutral substance. •NaCl is not the only ionic compounds that are salts. Others include: KCl, KBr, NH4NO3 •Indicators are used to test whether a substance is an acid or a base. •Phenolphthalein – common indicator, colorless in an acid; pink in a base. colorless in an acid pink in a base.