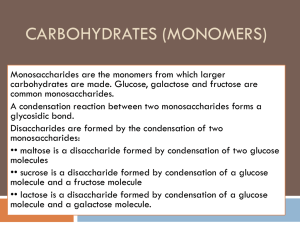
carbohydrates_
advertisement
CORE PRINCIPLES Biological molecules CARBOHYDRATES: Learning objective to be able to: • Identify the elements that make up carbohydrates • To describe how monosacharides are the basic molecular units of carbohydrates. • Explain how the condensation of monosacharides forms disacharides • Which elements make up carbohydrates? • Carbon, hydrogen and oxygen • What are carbohydrates needed for? • Energy • Where can we find carbohydrates? • Pasta, potatoes, bread etc • Give the chemical formula of a well known carbohydrate • C6H12O6 - glucose Carbohydrates include monomers, dimers and polymers. Carbohydrates sugars Monosaccharides (monomers) Disaccharides (dimers) Polysaccharides (polymers) eg glucose fructose ribose eg sucrose maltose lactose eg starch cellulose glycogen Monosaccharides • These all have the formula (CH2O)n, where n can be 3-7. • The most common and important monosaccharide is glucose, where n=6 • This is a six-carbon or hexose sugar, so has the formula C6H12O6. Its structure is: 6 CH OH 2 C5 O H H C4 HO OH C3 H CH2OH O H C1 H OH C2 OH Or more simply: HO OH Isomers of glucose • They have the same molecular formula (C6H12O6), but different structural formulae. • These isomers include • Fructose • Galactose • Mannose αβ-- Glucose CH2OH H H C O C OH H C OH C C OH H OH α- Glucose Galactose CH2OH OH H H C O C OH H C OH H C C OH H OH Fructose Pentose monosaccharides • Common five-carbon, or pentose sugars (where n = 5, C5H10O5) include • Ribose • Deoxyribose –(found in nucleic acids and ATP) • Ribulose Disaccharides • Disaccharides are formed when two monosaccharides are joined together by a glycosidic bond. • The reaction involves the formation of a molecule of water (H2O) CH2 OH CH2 OH O OH O OH OH glucose + glucose = maltose OH CH2OH C5 O H H H C4 C1 OH H HO OH C3 C2 H OH 6 CH2OH C5 O H H H C4 C1 OH H HO OH C3 C2 H OH 6 CH2OH CH2OH 6 C5 O H C5 O H H H H H C4 C1 C4 C1 H OH H HO OH O OH C3 C2 C3 C2 H H OH OH glycosidic bond 6 + H 2O •This shows two glucose molecules joining together to form the disaccharide maltose. •Because this bond is between carbon 1 of one molecule and carbon 4 of the other molecule it is called a 1-4 glycosidic bond. •Bonds between other carbon atoms are possible, leading to different shapes, and branched chains. Polymerisation • When molecules are joined together this is called polymerisation • Because a small molecule is lost, (usually water) this is called a condensation reaction • The reaction that joins two monosaccharides together is therefore called a – condensation polymerisation reaction Hydrolysis • Breaking apart the disaccharide would require a water molecule • This is called a hydrolysis reaction In general: •polymerisation reactions are condensations •breakdown reactions are hydrolysis Three common disaccharides • Maltose (or malt sugar) is glucose 1-4 glucose. • Sucrose (or cane sugar) is glucose 1-2 fructose. • Lactose (or milk sugar) is galactose 1-4 glucose. Summary of Carbohydrates • Carbon atoms bond strongly to each other forming chains and rings. • Carbohydrates include monosaccharides, disaccharides and polysaccharides. They can act as an energy source or provide structural support. • Monosaccharides include glucose, fructose and galactose. They have the same chemical formula but different chemical structures ~ we call them isomers. • Monosaccharides can become linked together by a glycosidic bond to form disaccharides such as maltose, sucrose and lactose, and polysaccharides, such as starch and cellulose. • Polysaccharides can perform structural roles, for example cellulose in plant cells, and energy storage roles, for example starch in plants and glycogen in animals.