Chemistry in Biology
advertisement

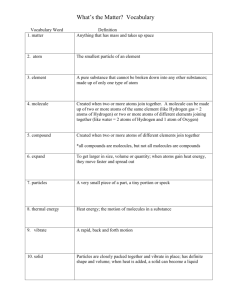
Chemistry in Biology 6 The Big Idea Atoms are the foundation of biological chemistry and the building blocks of all living things. Section 6.1: Main Idea Matter is composed of tiny particles called atoms. 6.1 ATOMS, ELEMENTS, and COMPOUNDS I. Chemistry is the study of matter. A. Matter is anything that takes up space and has mass. -Everything in the universe has matter. -Matter is composed of atoms. B. Mass is the amount of matter that an object has. -Mass and weight are not the same thing. C. Weight is the pull of gravity on an object 6.1 ATOMS, ELEMENTS, and COMPOUNDS II. Composition of Matter A. Elements—pure substances that cannot be broken down into simpler kinds of matter • Made of one type of atom • More than 100 elements (92 naturally occurring) • 90% of the mass of an organism is composed of 4 elements (oxygen, carbon, hydrogen, and nitrogen) • Each element has a unique chemical symbol -consists of 1-2 letter -first letter is always capitalized -Ex: O, C, F, Al, Ag, Mg 6.1 ATOMS, ELEMENTS, and COMPOUNDS B. Atoms—the simplest particle of an element that still has all the properties of that element • Atoms are so small that their true structure cannot be observed. Our understanding of the structure of atoms is based on scientific models. 6.1 ATOMS, ELEMENTS, and COMPOUNDS C. Parts of an atom •The nucleus of an atom contains two types of particles: 1. Proton—positively charge 2. Neutron—has no charge, “neutral” •The nucleus contains most of the mass of the atoms •All atoms of a given element have the same number of protons 6.1 ATOMS, ELEMENTS, and COMPOUNDS • The third particle of the atom is the electron which is located outside the nucleus in energy levels (electron clouds). -first energy level holds 2 electrons -second holds 8 electrons • Electrons have a negative charge. • Number of protons is balanced by an equal number of electrons therefore there is no charge of the atom. • All atoms have this same basic structure. Structure of an Atom 6.1 ATOMS, ELEMENTS, and COMPOUNDS D. Neutrons •The number of neutrons varies slightly among atoms for the same element. •Atoms of the same element that have different numbers of neutrons are referred to as isotopes of that element 6.1 ATOMS, ELEMENTS, and COMPOUNDS • Changing the number of neutrons does not change the overall charge of the atom but can affect the stability of the nucleus causing it to decay or break apart. Such isotopes give off radiation and referred to as “radioactive isotopes.” 6.1 ATOMS, ELEMENTS, and COMPOUNDS E. Atomic Mass and Atomic Number •Protons and neutrons each have a mass of 1 amu (atomic mass unit) or Dalton. •The atomic mass of an element is equal to the number of protons plus the number of neutrons in the nucleus of the atom. •The atomic number of an element is equal to the number of protons. The Periodic Table 6.1 ATOMS, ELEMENTS, and COMPOUNDS F. Periodic Table •The elements are arranged by their atomic number on the Periodic Table. •The horizontal rows are called periods and tell the number of energy levels. •The vertical groups are called families and tell the outermost number of electrons. 6.1 ATOMS, ELEMENTS, and COMPOUNDS G. Compounds • Compounds are pure substances formed when two or more different elements combine. -Ex: H20, CO2, and C6H12O6 • Compounds are always formed from a specific combination of elements in a fixed ratio. • Compounds cannot be broken down into simpler compounds or elements by physical means. -can be broken down by chemical means into simpler compounds or into their original elements -H2O can be broken down into hydrogen gas and oxygen gas 6.1 ATOMS, ELEMENTS, and COMPOUNDS • Elements can undergo chemical reactions to combine with other elements in order to become stable. • Stable elements have their outer energy level filled-meaning they have 8 electrons 6.1 ATOMS, ELEMENTS, and COMPOUNDS H. Chemical Formulas • Subscript after a symbol tell the number of atoms of each element • H20 has 2 atoms of hydrogen & 1 atom of oxygen • Coefficients before a formula tell the number of molecules • 3O2 represents 3 molecules of oxygen or (3x2) 6 atoms of oxygen 6.1 ATOMS, ELEMENTS, and COMPOUNDS III. CHEMICAL BONDS A. The force that holds atoms together is known as a chemical bond. • Electrons are directly involved in the formation of chemical bonds. -They travel around the nucleus of an atom in areas called energy levels. -Each energy levels has a specific number of electrons that it can hold at any time -The first energy level which is closest to the nucleus can hold up to 2 electrons. -The second energy level can hold 8 electrons. 6.1 ATOMS, ELEMENTS, and COMPOUNDS B.Types of Chemical Bonds 1. Covalent bond—forms when atoms share one or more pairs of electrons •A molecule is a compound in which the atoms are held together by covalent bonds. •Can be a single, double, or triple bond depending on number of pairs of electrons shared. Covalent Bond 6.1 ATOMS, ELEMENTS, and COMPOUNDS 2. Ionic Bond—forms when atom gives up electrons and another receives electrons in order to become stable • Electrical attraction between two oppositely charged atoms or groups of atoms called ions. • Most ionic compounds are crystalline at room temperature and have higher melting points than molecular compounds formed by covalent bonds. 6.1 ATOMS, ELEMENTS, and COMPOUNDS • Sodium and Chlorine -both atoms are unstable -to become stable, sodium gives away 1 electron and becomes positive, chlorine receives 1 electron and becomes negative 6.1 ATOMS, ELEMENTS, and COMPOUNDS 6.1 ATOMS, ELEMENTS, and COMPOUNDS • Some atoms tend to donate or accept electrons more easily than other atoms. • The elements identified as metals tend to donate electrons. • The elements identified as nonmetals tend to accept electrons. 6.1 ATOMS, ELEMENTS, and COMPOUNDS 3. van der Waals Forces • Defined as the attraction between molecules • This occurs when molecules come close together, and the attractive forces between slightly positive and negative regions pull on the molecules and hold them together. • The strength of the attraction depends on the size of the molecule, its shape, and its ability to attract electrons • Important in biological processes. Section 6.2: Main Idea Chemical reactions allow living things to grow, develop, reproduce, and adapt. 6.2 Chemical Reactions I. Reactants and Products A. Chemical Reactions • A chemical reaction is the process by which atoms or groups of atoms in substances are reorganized into different substances. • Clues that a chemical reaction has taken place include the production of heat or light, and formation of a gas, liquid, or solid. • Chemical bonds are broken and formed during chemical reactions 6.2 Chemical Reactions B. Chemical Equations • Chemical formulas describe the substances in the reaction and arrows indicate the process of change. • Reactants are the starting substances, on the left side of the arrow. • Products are the substances formed during the reaction, on the right side of the arrow. • Reactants Products 6.2 Chemical Reactions • Glucose and oxygen react to form carbon dioxide and water. • Is this equation written correctly? 6.2 Chemical Reactions C. Balancing Equations •The law of conservation of mass states matter cannot be created or destroyed. •The number of atoms of each element on the reactant side must equal the number of atoms of the same element on the product side. Balancing Equations—Practice 1.Fe + Cl2 FeCl3 1. 2Fe + 3Cl2 2FeCl3 2.H2 + O2 H2O 2. 2H2 + O2 2H2O 3.C2H6 + O2 CO2 + H2O 3. 2C2H6+ 7O2 4CO2 + 6H2O 4.CO2 + H2 CH4 + H2O 4. CO2 + 4H2 CH4 + 2 H2O 5.Al + O2 Al2O3 5. 4Al + 3O2 2Al2O3 Bell-Ringer 1. QUIETLY find your seat. 2. Sit down and prepare for the quiz. 3. Clear your desk of everything except a writing utensil. 4. Failure to do so will result in a 10pt deduction on your quiz. Quiz 1. A ____________is the process by which atoms or groups of atoms in substances are reorganized into different substances. 2. _____________ is/are the attraction between molecules. 3. _____________ states matter cannot be created nor destroyed. 4. Subscripts tell the number of _____________ per element. 5. Coefficients tell the number of ______________. 6.2 Chemical Reactions II. ENERGY A.Energy is defined as the ability to do work or cause a change. B.States of Matter: • Solid—particles are packed tightly and move slowly • Liquid—particles move freely and take the shape of the container • Gas—particles move rapidly and have no definite shape 6.2 Chemical Reactions C. Energy of Reactions • The activation energy is the minimum amount of energy needed for reactants to form products in a chemical reaction. 6.2 Chemical Reactions Exothermic Reaction Endothermic Reaction 6.2 Chemical Reactions D. Energy Changes in Chemical Reactions • This reaction is exothermic and released heat energy. • The energy of the product is lower than the energy of the reactants. 6.2 Chemical Reactions • This reaction is endothermic and absorbed heat energy. • The energy of the products is higher than the energy of the reactants. 6.2 Chemical Reactions III. ENZYMES A. An enzyme is a biochemical catalyst. • A catalyst is a substance that lowers the activation energy needed to start a chemical reaction. • It does not increase how much product is made and is not used up in the reaction. 6.2 Chemical Reactions • The reactants that bind to the enzyme are called substrates. • The specific location where a substrate binds on an enzyme is called the active site. • The active site changes shape and forms the enzyme-substrate complex, which helps chemical bonds in the reactants to be broken and new bonds to form. 6.2 Chemical Reactions B. Enzyme Action 1.Enzyme and substrate link together. 2.Chemical reaction occurs 3.Substrate becomes something new and the enzyme is let go. 4.The enzyme can be reused. Enzyme Action 6.2 Chemical Reactions • Factors such as pH, temperature, and other chemicals affect enzyme activity. • When enzymes are exposed to one of these factors, they may become denatured. -Denaturing causes the enzyme to not be as effective or possibly quit working altogether. Examples of Biological Enzymes • http://www.woisd.net/moodle/mod/resou rce/view.php?id=43 Section 6.3: Main Idea The properties of water make it well suited to help maintain homeostasis in an organism. 6.3 Water and Solutions I. Water’s Polarity A. Molecules that have an unequal distribution of charges are called polar molecules. • Polar molecules have one side that has a postive charge and the other has a negative charge. • Water is a polar molecule. B. Water molecules are held together by weak hydrogen bonds. • A hydrogen bond is a weak interaction involving a hydrogen atom and a fluorine, oxygen, or nitrogen atom. • Hydrogen bonds are important in biological processes. 6.3 Water and Solutions 6.3 Water and Solutions II. Properties of Water 1. Cohesion is the attractive force between particles of the same kind. This property allows water to bulge from the top of a filled glass without spilling over. 2. Adhesion is the attractive force between unlike substances. •Together these properties make it possible for water to move upwards against the force of gravity in a narrow tube. •This phenomenon is called capillarity. 6.3 Water and Solutions III. Mixtures with Water A.Mixture--a combination of two or more substances in which each substance retains its individual characteristics B.Homogenous mixture--one that has a uniform composition throughout; a solution C.Solvent--a substance in which another substance is dissolved (Ex: tea) D.Solute--the substance that is dissolved in the solvent (Ex: sugar) Food coloring dissolved in water forms a homogenous mixture. 6.3 Water and Solutions E. In a heterogeneous mixture, the components remain distinct. •In a suspension, the components will eventually settle to the bottom. •In a colloid, the particles will not settle over time. Examples include fog, smoke, butter, mayonnaise, milk, paint, and ink. 6.3 Water and Solutions F. Concentration—the measure of how much of a substance is dissolved G. Saturated solution—one in which nothing else can dissolve H. Aqueous solution—water is the solvent 6.3 Water and Solutions IV. Acids and Bases A. Substances that release hydrogen ions (H+) when dissolved in water are called acids. B. Substances that release hydroxide ions (OH–) when dissolved in water are called bases. 6.3 Water and Solutions 6.3 Water and Solutions C. The pH Scale •The measure of concentration of H+ in a solution is called pH. -Ranges from 0-14 with 7 being neutral •Acidic solutions have pH values lower than 7. -have a sour taste •Basic solutions have pH values higher than 7. -have a bitter taste •Buffers are mixtures that can react with acids or bases to keep the pH within a particular range. -help neutralize a body’s pH level 6.3 Water and Solutions Section 6.4: Main Idea Organisms are made up of carbon-based molecules. 6.4 The Building Blocks of Life Organic Chemistry The element carbon is a component of almost all biological molecules. 6.4 The Building Blocks of Life • Carbon has four electrons in its outermost energy level. • One carbon atom can form four covalent bonds with other atoms. • Carbon compounds can be in the shape of straight chains, branched chains, and rings. 6.4 The Building Blocks of Life Macromolecules • Carbon atoms can be joined to form carbon molecules. • Macromolecules are large molecules formed by joining smaller organic molecules together. • Polymers are molecules made from repeating units of identical or nearly identical compounds linked together by a series of covalent bonds. 6.4 The Building Blocks of Life Carbohydrates • Compounds composed of carbon, hydrogen, and oxygen in a ratio of one oxygen and two hydrogen atoms for each carbon atom— (CH2O)n 6.4 The Building Blocks of Life • Values of n ranging from three to seven are called simple sugars, or monosaccharides. • Two monosaccharides joined together form a disaccharide. • Longer carbohydrate molecules are called polysaccharides. 6.4 The Building Blocks of Life Lipids Molecules made mostly of carbon and hydrogen A triglyceride is a fat if it is solid at room temperature and an oil if it is liquid at room temperature. 6.4 The Building Blocks of Life • Lipids that have tail chains with only single bonds between the carbon atoms are called saturated fats. • Lipids that have at least one double bond between carbon atoms in the tail chain are called unsaturated fats. • Fats with more than one double bond in the tail are called polyunsaturated fats. 6.4 The Building Blocks of Life Proteins • A compound made of small carbon compounds called amino acids • Amino acids are small compounds that are made of carbon, nitrogen, oxygen, hydrogen, and sometimes sulfur. 6.4 The Building Blocks of Life • Amino acids have a central carbon atom. • One of the four carbon bonds is with hydrogen. • The other three bonds are with an amino group (–NH2), a carboxyl group (– COOH), and a variable group (–R). 6.4 The Building Blocks of Life • The number and the order in which the amino acids are joined define the protein’s primary structure. • After an amino acid chain is formed, it folds into a unique three-dimensional shape, which is the protein’s secondary structure, such as a helix or a pleat. 6.4 The Building Blocks of Life • Nucleic acids are complex macromolecules that store and transmit genetic information. • Nucleic acids are made of smaller repeating subunits called nucleotides, composed of carbon, nitrogen, oxygen, phosphorus, and hydrogen atoms 6.4 The Building Blocks of Life