Acute MI: Public Health Issues
advertisement
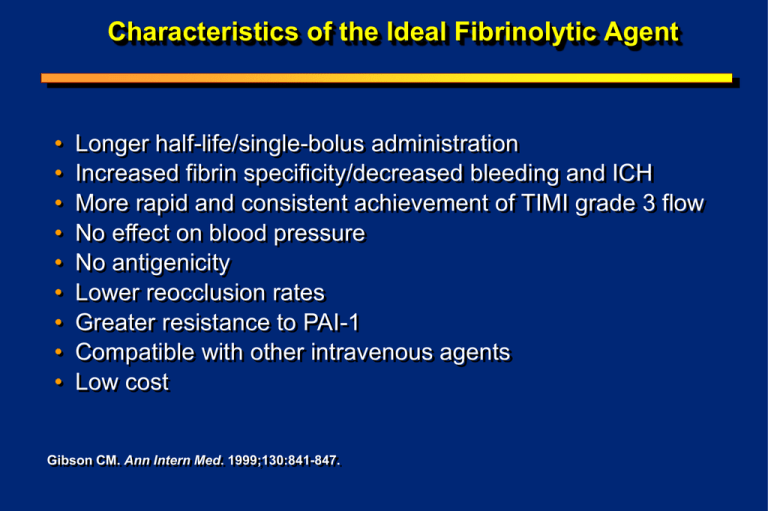
Characteristics of the Ideal Fibrinolytic Agent • • • • • • • • • Longer half-life/single-bolus administration Increased fibrin specificity/decreased bleeding and ICH More rapid and consistent achievement of TIMI grade 3 flow No effect on blood pressure No antigenicity Lower reocclusion rates Greater resistance to PAI-1 Compatible with other intravenous agents Low cost Gibson CM. Ann Intern Med. 1999;130:841-847. Molecular Structures of Fibrinolytics t-PA (alteplase) n-PA (lanoteplase) TNK t-PA (tenecteplase) r-PA (reteplase) Fibrin Specificity SK r-PA / n-PA t-PA TNK-tPA Fibrinolytics in Development: Comparative Overview Tenecteplase (TNK-tPA) Lanoteplase (n-PA) 20 37 Single bolus Single bolus Provides patientspecific weightbased dosing Yes Fibrin specificity PAI-1 resistance Half-life (minutes) Dosing Antigenic Plasminogen activation Staphylokinase Saruplase 6 2 boluses 30 min apart 9 Bolus + 60min infusion Yes ?? ?? +++ + +++ + Increased ?? ?? ?? No No Yes Yes Direct Direct Indirect Direct TNK-tPA: Molecular and Biochemical Properties Kringle 1 T Domain Asn for Thr at N Domain Gln for Asn at 117 : Increased fibrin specificity Kringle 2 103: Reduces clearance; single bolus EGF K Domain Ala-Ala-Ala-Ala for Lys-His-Arg at 296-299: More resistant to PAI 1, enzyme which breaks down lytic agents Finger NH2 HOOC Percent of Patients TIMI-10B: TIMI Grade Flow at 90 Minutes 100 90 80 70 60 50 40 30 20 10 0 19 16 22 22 TIMI grade 2 TIMI grade 3 63 63 66 TNK-tPA 40mg TNK-tPA 50mg 55 t-PA TNK-tPA 30mg n=312 n=304 *P=0.047, TNK-tPA 30 mg vs t-PA; all others, P=NS. Cannon CP, Gibson CM, McCabe CH et al. Circulation. 1998;98:2805-2814. n=146 n=76 TIMI-10B: TIMI Grade 3 Flow at 90 Minutes by Dose/Weight Percent TIMI Grade 3 Flow at 90 Minutes 70 60 50 40 No further improvement In flow above 0.53 mg/kg 30 20 P=0.028 10 0 0.2 0.3 0.4 0.5 0.6 TNK-tPA Dose/Weight (quintiles, mg/kg, mean) Cannon CP, Gibson CM, McCabe CH et al. Circulation. 1998;98:2805-2814. 0.7 TIMI-10B: Relationship Between TIMI Frame Count & Dose / Weight High Dose = 0.52 to 1.24 mg / Kg. Dose of 0.53 mg / kg selected for ASSENT 2 based upon logistic regression of CTFC p = 0.007 40 38 35 TIMI Frame Count 35 20 35 31 31 30 25 p = 0.002 40 Low Med High Low Med High 0.20 to 0.40 to 0.52 to 0.20 to 0.40 to 0.52 to 0.39 0.51 1.24 0.39 0.51 1.24 mg/kg mg/kg mg/kg mg/kg mg/kg mg/kg N=166 N=174 N=171 N=107 N=127 15 10 5 N=104 0 Culprit Gibson CM, et al. Am J Cardiol. 1999;84:976-980. All 3 Arteries Pathophysiology of Improved Flow With Weight Optimized TNK Dosing: Weight Optimizing Reduces Thrombus Burden & Improves Percent Stenosis p = 0.06 % Patients 35 77 34.3 76 29 30 25 25 20 15 Low Med High 10 5 74 73 72 71 N=166 N=174 N=171 0 75.7 75 % Stenosis 40 p = 0.03 70 71.4 Low High N=173 N=173 69 Thrombus Gibson CM, et al. Am J Cardiol. 1999;84:976-980. % Stenosis TIMI-10B: Weight Optimized Dosing “Facilitates Adjunctive PCI” 35 p = 0.05 29 TIMI Frame Count 30 Dose of 0.53 mg / kg selected for ASSENT 2 based upon logistic regression of Frame Count data 24 25 19 20 15 10 Low Med High 0.20 to 0.40 to 0.52 to 0.39 0.51 1.24 mg/kg mg/kg mg/kg N=46 N=39 N=20 5 0 Post PTCA Gibson CM, et al. Am J Cardiol. 1999;84:976-980. ASSENT-2: Similar Incidence of Stroke for TNK-tPA and t-PA TNK-tPA (n=8,461) t-PA (n=8,488) Relative Risk (95% CI) Total stroke (%) 1.78 1.66 0.555 Intracranial hemorrhage (%) 0.93 0.94 1.07 (0.856-1.349) 0.991 (0.727-1.350) Ischemia 0.72 0.64 1.13 (0.787-1.632) 0.514 With hemorrhagic conversion (%) 0.07 0.09 0.752 (0.261-2.168) 0.790 Unknown type (%) 0.13 0.08 1.576 (0.611-4.065) 0.358 ASSENT-2 Investigators. Lancet. 1999;354:716-722. P Value 1.000 Confusion in Reperfusion: The “Old Old” (>75 yrs) • Highest risk for complications, but potentially have the most to gain from treatment • Understudied in randomized trials, but now over 1/3 rd of MIs are > 75 years • Heterogeneous group, multiple risk factors at play, potential for interactions • Tend to present late CM Gibson, GW symposium, AHA 2000 ICH Risk is Eight Time Higher in Elderly Females following t-PA 12 Adjusted OR 10 8 6 4 2 0 M<65 F<65 M 65-74 Gurwitz et al. 1998 Annals Int Med. 129; 597-604. F 65-74 M>75 F>75 Unadjusted Dose Rises Rapidly in People of Low Body Weight 3 tPA Fixed dose TNK Dose (mg/kg) 2.5 2 1.5 1 0.5 0 20 30 40 50 60 70 Weight (kg) CM Gibson, GW symposium, AHA 2000 80 90 100 110 New Answers to the Old Old Question • How might weight optimized dosing of TNK favorably alter outcomes in this very high risk group of low body weight women over 75 years of age? CM Gibson, 2000 Weight Optimizing Reduces ICH Rates : ASSENT II P=0.18 3 % of Patients 2.62 2.5 2 1.78 1.77 1.72 1.44 1.5 1.41 1 0.5 TNK tPA TNK tPA TNK tPA 0 >75 H Barron AHA 1999; Circulation 1999; 100: I-1 Female <67kg Weight Optimizing Reduces ICH Rates : ASSENT II ICH Rates Among Women > 75 Years who are < 67 Kg in ASSENT II Patients (%) P <0.05 3.02 1.14 TNK-tPA H Barron AHA 1999; Circulation 1999; 100: I-1 tPA Weight Optimizing Reduces ICH Rates : ASSENT II Multivariate Model Odds Ratio 95% Confidence Ratio Age 1.788 (1.529, 2.091) Weight 0.760 (0.668, 0.864) Diastolic Blood Pressure 1.024 (1.013, 1.036) Hypertension 1.513 (1.096, 2.088) TNK Treatment by high-risk females* 0.300 (0.092, 0.977) * Females >75 years and <67 kgs H Barron AHA 1999; Circulation 1999; 100: I-1 Weight Optimizing TNK Reduces the ICH Risk Among High Risk Patients • In women > 75 years of age and < 67 kg weight optimizing TNK reduces the risk of ICH by 70% compared with t-PA H Barron AHA 1999; Circulation 1999; 100: I-1 Weight Optimized Dosing of TNK Improves Rate of Opening by 60 Minutes % of Patients Dose of 0.53 mg / kg selected for ASSENT 2 based upon logistic regression of Frame Count data 84 82 80 78 76 74 72 70 68 66 64 p = 0.02 vs low dose 81 70 82 Med High N=178 N=174 Low N=177 Open by 60 Min. Gibson CM, et al. Am J Cardiol. 1999;84:976-980. Weight Optimized Dosing of TNK Improves Microvascular Function • Improved flow in all 3 arteries (even in those without a stenosis) • In a multivariate model correcting for % stenosis, thrombus & early opening by 60 minutes, weight optimized dose group was 5 frames faster • Following relief of the stenosis by PCI, weight optimized dose arm was faster Gibson CM, et al. Am J Cardiol. 1999; 84:976-980. ASSENT-2 Trial Design Patients with AMI and ST-segment elevation, symptom onset 6 h (n = 16,950; 1,021 hospitals) Objective: demonstrate “equivalence” Primary endpoint: all-cause 30-day mortality Aspirin (150-325 mg) IV heparin >67 kg: 5000-U bolus, 1000 U/h <67 kg: 4000-U bolus, 800 U/h Randomization t-PA-accelerated regimen (weight-adjusted) Adapted from ASSENT-2 Investigators. Lancet. 1999;354:716-722. TNK-tPA single bolus (weight-adjusted) Understanding Equivalence Superiority: Does the 95% CI contain zero? 1% 0% +1% Equivalence: Does the 95% CI lie between 1% and +1%? 1% To left of 1% is clinically meaningful CM Gibson, 2000 0% Between 1% and +1% is not clinically meaningful +1% To right of 1% is clinically meaningful Comparison Among Equivalency Analyses for 30-Day Mortality Mortality (%) InTIME-2 ASSENT-2 GUSTO-III n-PA t-PA 6.77 6.60 TNK-tPA t-PA 6.16 6.18 r-PA t-PA 7.47 7.24 Absolute Difference (95% CI) Other Better t-PA Better P Value for Equivalence 0.17 (1.0, 0.68) 0.02 (0.59, 0.62) 0.047 0.006 0.23 (1.11, 0.66) NS -1 0 +1 ASSENT-2 Investigators. Lancet. 1999;354:716-722; Adapted from GUSTO-III Investigators. N Engl J Med. 1997;337:1118-1123. Adapted from Giugliano RP, et al. Circulation. 1999;100:I-651. Major Trials Comparing 30- or 35-Day Mortality Among Fibrinolytics Superiority: Equivalency: 12 2P<0.00001 P=NS 12 Mortality % 10 P=0.001 P=NS P=NS P=0.0003 Superiority Demonstrated 9.5 7.4 P=NS P=0.006 7.2 9 7.5 6.6 6.77 6.3 6 P=NS P=0.047 Equivalency Demonstrated 9.2 8 6.15 6.17 * 4 Agents 2 P=NS P=NS Placebo SK t-PA t-PA r-PA GUSTO-I GUSTO-III SK SK r-PA t-PA n-PA t-PA TNK tPA 0 ISIS-2 CM Gibson, 2000 INJECT InTIME-2 ASSENT- 2 *Higher ICH rate for n-PA (0.62% vs 1.13%; P=0.003). *Lower major bleeds for TNK-tPA (4.7% vs 5.9%; P=0.0002). ASSENT-2: 30-Day Mortality By Age and Sex TNK-tPA (n=8,461) t-PA (n=8,488) Relative Risk (95% CI) P Value <75 4.6 4.3 1.063 (0.915-1.235) 0.425 >75 17.4 19.3 0.903 (0.754-1.081) 0.286 5.0 4.8 1.039 (0.894-1.209) 0.627 10.0 10.6 0.943 (0.784-1.134) 0.563 Age (years) Sex Male Female ASSENT-2 Investigators. Lancet. 1999;354:716-722. ASSENT-2: 30-Day Mortality By Infarct Location, Previous AMI, or Previous CABG; Killip Class; and History of Hypertension or Diabetes Infarct location Anterior Other TNK-tPA (n=8,461) t-PA (n=8,488) Relative Risk (95% CI) P Value 8.0 5.0 8.2 4.8 0.975 (0.830-1.146) 1.026 (0.865-1.218) 0.789 0.783 9.8 8.6 1.137 (0.897-1.441) 0.318 5.5 5.7 0.965 (0.843-1.105) 0.609 9.8 6.0 7.7 6.1 1.280 (0.776-2.111) 0.987 (0.875-1.115) 0.406 0.844 4.7 13.5 29.0 51.4 4.8 13.4 24.5 61.1 0.983 (0.851-1.134) 1.011 (0.797-1.281) 1.185 (0.740-1.899) 0.842 (0.556-1.273) 0.818 0.944 0.516 0.477 8.0 5.0 7.6 5.2 1.050 (0.888-1.241) 0.962 (0.816-1.135) 0.578 0.657 8.8 5.6 8.7 5.7 1.002 (0.786-1.278) 0.993 (0.868-1.136) 1.000 0.942 Previous AMI Yes No Previous CABG Yes No Killip Class I II III IV Hypertension Yes No Diabetes Yes No ASSENT-2 Investigators. Lancet. 1999;354:716-722. ASSENT-2: Improved Survival for TNK-tPA in Late-Treated Patients TNK-tPA (n=8,461) Total population (%) t-PA Relative Risk (n=8,488) (95% CI) TNK-tPA Better t-PA Better P Value 6.16 6.18 1.00 (0.89, 1.12) 0.975 0-2 (%) 5.0 4.9 1.017 (0.799 - 1.296) 0.897 >2-4 (%) 6.3 5.5 >4 (%) 7.0 9.2 Time to therapy (h) 1.157 (0.970, 1.379) 0.766 (0.617. 0.952) ASSENT-2 Investigators. Lancet. 1999;354:716-722. 0.106 0.018 0.4 1 1.4 Percent Changes in Parameters (median) TIMI-10B: Clinical Evidence of Increased Fibrin Specificity in TNK-tPA Fibrinogen 0 40 30 30 40 50 Plasminogen 0 40 -10 50 30 TNK-tPA 50 -10 30 40 -20 -20 30 40 40 50 50 50 TNK-tPA -30 A 30 -30 -40 -40 A -50 A A alteplase (t-PA) A -50 alteplase (t-PA) A -60 0 1 3 6 0 Hours Post-Dose Cannon CP, Gibson CM, McCabe CH et al. Circulation. 1998;98:2805-2814. 1 3 Hours Post-Dose 6 ASSENT-2: Significantly Fewer Noncerebral Bleeding Events With TNK-tPA TNK-tPA (n=8,461) t-PA (n=8,488) P Value Total bleeds (%) 26.4 29.0 0.0003 Major bleeds (%) 4.7 5.9 0.0002 Minor bleeds (%) 21.8 23.0 0.0553 Units transfused Any 1-2 units >2 units 4.3 2.6 1.7 5.5 3.2 2.2 0.0002 - ASSENT-2 Investigators. Lancet. 1999;354:716-722. How Does Weight Optimized Dosing Improve Patient Care? • Improves efficacy (earlier & better flow, less thrombus, less stenosis & better outcomes) in heavier patients • Improves safety (lower intracranial hemorrhage) in high risk patients CM Gibson, GW symposium, AHA 2000 Why Not Use a Fixed 40 mg Dose in All Patients? In heavy patients: These patients receive relatively less drug. Weight optimized dosing may improve rates of TIMI grade 3 flow & TIMI Frame Counts, and thereby lower mortality. In light patients: These patients receive relatively more drug. Weight optimized dosing may reduce the risk of serious bleeding events and reduce the risk of intracranial hemorrhage rate in lowerweight patients. CM Gibson, GW symposium, AHA 2000 Other Acute MI Regimens Use Weight Adjusted Dosing • • • • • Heparin Reopro Integrilin Aggrastat Low Molecular weight heparinoids • Dopamine, dobutamine • As TNK is combined with heparin & glycoprotein 2b3a inhibitors, safety & efficacy will hopefully be improved as a result of weight optimized dosing CM Gibson, GW symposium, AHA 2000 TNK-tPA Dosing Regimen Dosing categories are about 22 lb wide – Minimizes the possibility of dosing errors < 60 kg 61 - 70 kg 71 - 80 kg 81 - 90 kg > 90 kg (< 132 lbs) (133-154 lbs) (155-176 lbs) (177-198 lbs) (> 199 lbs) 6 mL 7 mL 8 mL 9 mL 10 mL Confusion in Reperfusion: Dose Errors • Do dose errors cause death ? Or • Does death cause dose errors ? CM Gibson, GW symposium, AHA 2000 Dosing Errors to be Studied • Timing of dose – No potential for error with a single bolus agent such as TNK • Duration of injection – Little or no real potential for error with a 5 second administration of TNK • Compatibility with other drugs – Little potential for error with TNK CM Gibson, GW symposium, AHA 2000 Two Common Questions Asked About the 50 mg Dose of TNK • Is the 50 mg dose safe? Is there an excess risk of intracranial hemorrhage and bleeding associated with 50 mg? • Is the 50 mg dose efficacious? Is 50 mg of TNK in heavy patients (I.e. those > 90 Kg) adequate? In other words, are very heavy patients being “under dosed”? CM Gibson, GW symposium, AHA 2000 Safety and Efficacy of 50 mg of TNK Differences Between TIMI 10B and ASSENT 2 • In TIMI 10B, 50 mg of TNK was given to patients of all weights • In ASSENT 2, 50 mg of TNK was given only to patients weighing over 90 Kg CM Gibson, GW symposium, AHA 2000 Wide Range of Weights Among Pts. Given 50 mg TNK in TIMI 10B >90 kg 22% <60 kg 11% 61-70 kg 17% 81-90 kg 25% 71-80 kg 25% CM Gibson, GW symposium, AHA 2000 • In TIMI 10B there were a small number of patients (78) who received 50 mg of TNK, the majority of whom were of lighter weights (under 90 Kg) • Only 22.4% of pts. in TIMI 10B weighed > 90 Kg, the weight required for therapy with 50 mg of TNK in ASSENT 2 & in clinical practice Differences Between TIMI 10B and ASSENT 2 in Examining the Safety and Efficacy of 50 mg of TNK • In TIMI 10B, only 78 patients received 50 mg of TNK • There were 3 intracranial hemorrhages • None of the patients in TIMI 10 B with an intracranial hemorrhage weighed over 90 Kg (mean wt. only 77.2 Kg)* • These 3 patients all weighed < 90 Kg, and none of them would have received 50mg as a weight adjusted dose in ASSENT 2 or in clinical practice • There were 9 major hemorrhages, and only 1 patient weighed over 90 Kg* • Thus, 89%* of all patients (8/9) who developed a major hemorrhages at the 50mg dose would not have received this dose had they been enrolled in the ASSENT 2 trial or treated in clinical practice • This was in part the motivation for weight adjusting the dose of TNK CM Gibson, GW symposium, AHA 2000 ASSENT-2: Outcomes With 50 mg TNK vs Lower Doses of TNK 7 6 50 mg < 50 mg P = 0.006 6.4 5 4.7 % 4 3 2 P = 0.06 1 0 1.07 0.57 ICH Alexander et al, AHA 2000 Death • Mortality and ICH were both lower with the 50 mg dose • The difference in outcome, however, is not significant when adjusting for multiple variables including weight Treatment With 50 mg TNK is Associated with low ICH Rates: Major Trials Comparing Intracranial Hemorrhage Rates Among Fibrinolytics P=0.003 1.2 1.13 P=NS P=NS 1 0.87 P =NS 0.94 0.93 0.91 ICH (%) 0.77 0.8 0.62 0.57* 0.6 2P<0.02 0.37 0.4 0.1 0.2 0 0 Placebo SK ISIS-2 SK r-PA INJECT t-PA r-PA GUSTO-III t-PA n-PA InTIME-II t-PA TNK tPA ASSENT-2 50 mg TNK in ASSENT 2 Califf RM, et al. Am Heart J. 1997;133:630-639; ISIS-2 Collaborative Group. Lancet. 1988;2:349-360; GUSTO III Investigators. N Engl J Med. 1997;337:1118-1123; INJECT. Lancet. 1995;346:329-336; Van de Werf F, et al. ACC 1999 CME Online. 1999;1-12. *Data on file, Genentech Treatment with 50 mg TNK is Associated with low Mortality Rates: Major Trials Comparing 30- or 35-Day Mortality Among Fibrinolytics P<0.00001 14 12 12 P=0.0003 9.2 Mortality % 10 9.53 9.02 7.24 7.47 7.4 8 6.3 6.6 6.77 6.15 6.17 4.78* 6 4 2 Placebo 0 SK ISIS-2 SK t-PA GUSTO-I SK r-PA INJECT t-PA r-PA GUSTO-III t-PA n-PA InTIME-II t-PA TNK tPA ASSENT- 2 50 mg TNK ASSENT 2 Califf RM, et al. Am Heart J. 1997;133:630-639; ISIS-2 Collaborative Group. Lancet. 1988;2:349-360; GUSTO III Investigators. N Engl J Med. 1997;337:1118-1123; INJECT. Lancet. 1995;346:329-336; Van de Werf F, et al. ACC 1999 CME Online. 1999;1-12. *Data on file, Genentech Is 50 mg of TNK Administered to Patients with an Estimated Weight > 90 Kg Both Safe & Effective? It is safe: Using the dose schedule in ASSENT 2 (I.e. 50 mg TNK for pts. with estimated wt. > 90 Kg): Risk of ICH in TIMI 10B: Risk of ICH in ASSENT 2: Risk of ICH in TIMI 10B & ASSENT 2 combined: 0.0%* 0.57%* 0.566%* It is effective: Risk of mortality was 4.78% in pts. treated with 50 mg in ASSENT 2, which compares favorably with the 6.18% rate among all patients in the ASSENT 2 study* The 4.78%* mortality observed among the subgroup of pts. treated with 50 mg of TNK is the lowest mortality rate observed among recent thrombolytic trials CM Gibson, GW symposium, AHA 2000 Therapeutic Margin of Weight Adjusted Dosing of TNK • Questions have been raised about the “margin for error” with weight optimized dosing of TNK • What if the estimated weight is off by 10 Kg (22 pounds) which would be one dose category? What if the weight estimate is off by 2 weight categories (20 Kg or 44 pounds)? • Is this safe? Dose this pose an undue risk of ICH? CM Gibson, GW symposium, AHA 2000 Low Body Weight As A Risk Factor For Adverse Outcomes • Low Body weight has been identified as a risk factor for adverse outcomes following thrombolytic administration, even when the dose is administered correctly • Low body weight is a risk factor for adverse outcomes in primary PTCA patients • Low body weight is a risk factor for adverse outcomes even among patients who receive no reperfusion strategy CM Gibson, GW symposium, AHA 2000 Low Body Weight As A Risk Factor For Adverse Outcomes •Thus, low body weight is a potential confounder in the analysis of dose errors. Low body weight patients may be more likely to receive excess dosing of a drug, but may simultaneously be at risk for adverse outcomes simply on the basis of their low body weight alone • The question becomes “Is it the low body weight of the patient or the dose error that resulted in an adverse outcome” ? • To answer this question body weight must be accounted for in the analysis of dose errors CM Gibson, GW symposium, AHA 2000 Margin of Safety with TNK Overdoses • Across all dose arms, if an overdose error of 1 to 2 dose categories (up to 20 Kg, 44 pounds) was made, the odds ratio for ICH and death were no different than in the trial as a whole in a multivariate model adjusting for patient weight •TNK has a broad therapeutic margin of safety and errors of estimating weight by up to 20 Kg or 44 pounds are well tolerated with no increased risk of ICH or death CM Gibson, GW symposium, AHA 2000 Association Between Recording of Weight on Case Report Form and Mortality 12 10.1% 10 Mortality 8 Recently concerns have been expressed regarding the need to weigh patients prior to administration of a thrombolytic agent. Dosing in ASSENT 2 was based upon either estimated or actual weights 6.16% 6 Patients with missing weights had a higher mortality 4 2 0 Trial as a Whole Weight Missing Is this a cause of a higher mortality, or is it a marker of a sicker patient or a patient who died before being weighed? Among rPA Treated Patients Missing Weight is Associated with Higher Mortality: A Textbook Case of Statistical Confounding Weight not recorded in 11% of INJECT Trial patients 25 Mortality (%) 19.5% 20 16.3% 15 10.2% 10 5 0 Deaths N = 242 > 60 Kg N = 48 N = 65 < 60 Kg Missing weight FDA PLA 95-1167, July 15, 1996, page 33 •Mortality highest if no weight recorded •No biologically plausible reason why there should be a higher mortality among patients receiving a fixed lytic dose in whom no weight was recorded • Likely explanation is statistical confounding: Missing weight is a marker of a sicker patient, or the patient died before weight obtained Weight Optimized Dosing of TNK: The Advantages •TNK dosing is optimized for the patient’s weight so that flow at 90 minutes after its administration is accelerated •Weight optimized dosing also improves flow after PTCA / stenting and “facilitates PCI” •Weight optimizing TNK dosing improves microvascular function and flow in all 3 arteries •While dose is optimized, the range of weights for a given dose is 22 pounds or 10 Kg, which may minimize dosing errors •TNK is a single dose agent, TNK is compatible with other medications such as heparin (other lytics may precipitate) •TNK is the only agent to demonstrate equivalency to tPA with an acceptable safety profile •Weight adjusted dosing of TNK is safe, efficacious, and has a favorable therapeutic margin of safety if errors are made •Indeed the safety profile is improved: the risk of bleeding and transfusion is reduced CM Gibson, GW symposium, AHA 2000





