Enthalpy of Formation – Heat of Formation
advertisement

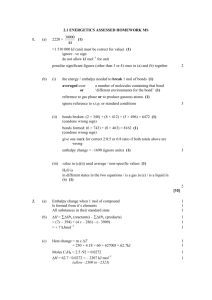
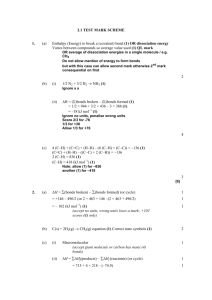
Enthalpy of Formation By Jamie Leopold Keep an eye out for the yummy food! In this PPT, you’ll learn all about… 1. What the enthalpy of formation (Heat of Formation) is. 2. What the standard enthalpy of formation is. 3. How to use the enthalpy of formation to calculate ∆H of a reaction what is enthalpy? – Enthalpy is the measure of the total energy of a thermo chemistry system or reaction. Enthalpy of Formation – Heat of Formation The enthalpy of formation is the heat released or absorbed, depending on what type of reaction it is, in a chemical reaction. The heat that is being either released or absorbed is doing so at a constant rate. If heat is released, then the sign of the enthalpy (or change in heat) will be negative. If heat is absorbed, then the sign will be positive. STANDARD ENTHALPY OF FORMATION The standard enthalpy of formation is the change in enthalpy (total energy, ie. heat absorbed or heat given off) to form a more complicated compound from its elements. For this to work, the elements must be in their most natural state (liquid, solid, gas, etc). A chart of where to find ALL of the standard enthalpy of formation numbers: http://chemistry.about.com/library/weekly /blheatform.htm - For example: how we can burn carbon in oxygen to form carbon dioxide. - The unit of measure for standard enthalpy of formation is either: ΔHf0 or ΔfH0 (measured in kJ/mol) - Thanks to the conservation of energy, standard enthalpy of formation AND enthalpy of formation can be used to calculate the heat absorbed/released in any chemical reaction. How to use the enthalpy of formation to calculate ∆H of a reaction: The basics - word equation for Hess’ Law: Enthalpy change = standard enthalpy of formation of product – standard enthalpy of formation of reactant General background Info: In an exothermic reaction: Energy of the reactants is greater than the energy of the products: H(reactants) > H(products) In an endothermic reaction: Energy of the reactants is less than the energy of the products: H(reactants) < H(products) From website #5 H = H(products) - H(reactants) “The standard state of any given substance is: 298 K (or 25 degrees C) and 1 atmosphere (1 atm) of pressure.” From website #5 Use: an example problem and how to do it step by step! Example by website 6: 1. If we break this equation up into its many different elements and parts we would get: 2 moles of B5H9 reacts with 12 moles of O2 to yield (or make) five moles of B2O3 and 9 moles of H2O. Now, as our formula says, we must add up the sums of our products and then subtract that from the sum of our reactants. Now the products: 2 mol B5H9 73.2 kj + 12 mol O2 0kj ---------------mol mol Website #2/6 Starting with the reactants: 5 mol B2O3 -1272.77kJ + 9 mol H2O ----------------mol -241.82 KJ -------------mol Part 2: 5 mol B2O3 -1272.77kJ + 9 mol H2O -241.82 KJ -----------------mol mol - 2 mol B H 73.2 kj + 12 mol O2 0kj --------------mol mol - 2 mol B H 73.2 kj + 12 mol O2 0kj --------------mol mol 5 9 Now, its time to cross out! 5 mol B2O3 -1272.77kJ + 9 mol H2O -241.82 KJ -----------------mol mol 5 9 You’re now left with only the numbers: You multiply the # of moles by the kJ/mol Reactants: 5 x -1272.77 = -6363.85 9 x -241.82 = -2176.38 Products: 2 x 73.2 = 146.4 12 x 0 = 0 Then you add and subtract them, like this: (-6363.85 + -2176.38) – 146.4 = -8686.6 kJ final answer!! BIBLIOGRAPHY: 1. http://chemed.chem.wisc.edu/chempaths/GenChemTextbook/Standard-Enthalpies-of-Formation-551.html 2. http://chemistry.about.com/library/weekly/blheatform.htm 3. http://www.mikeblaber.org/oldwine/chm1045/notes/Energy/HeatF orm/Energy05.htm 4. http://en.wikipedia.org/wiki/Standard_enthalpy_of_formation 5. http://www.ausetute.com.au/enthchan.html 6. http://www.chem.tamu.edu/class/majors/tutorialnotefiles/enthalp y.htm 7. http://en.wikipedia.org/wiki/Enthalpy 8. If you want the website for the great food: www.foodporn.com