Honors Chemistry Review Sheet: Intro, Moles, Nomenclature
advertisement
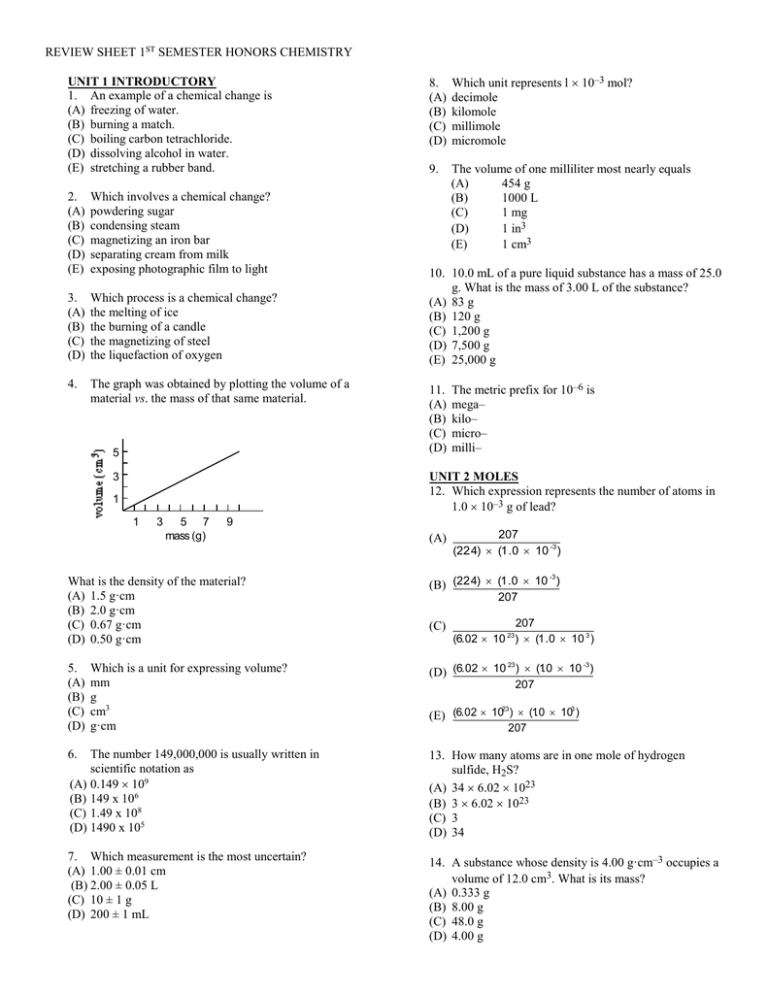
REVIEW SHEET 1ST SEMESTER HONORS CHEMISTRY UNIT 1 INTRODUCTORY 1. An example of a chemical change is (A) freezing of water. (B) burning a match. (C) boiling carbon tetrachloride. (D) dissolving alcohol in water. (E) stretching a rubber band. 2. (A) (B) (C) (D) (E) Which involves a chemical change? powdering sugar condensing steam magnetizing an iron bar separating cream from milk exposing photographic film to light 3. (A) (B) (C) (D) Which process is a chemical change? the melting of ice the burning of a candle the magnetizing of steel the liquefaction of oxygen 4. The graph was obtained by plotting the volume of a material vs. the mass of that same material. 5 Which unit represents l 10–3 mol? decimole kilomole millimole micromole 9. The volume of one milliliter most nearly equals (A) 454 g (B) 1000 L (C) 1 mg (D) 1 in3 (E) 1 cm3 10. 10.0 mL of a pure liquid substance has a mass of 25.0 g. What is the mass of 3.00 L of the substance? (A) 83 g (B) 120 g (C) 1,200 g (D) 7,500 g (E) 25,000 g 11. (A) (B) (C) (D) The metric prefix for 10–6 is mega– kilo– micro– milli– UNIT 2 MOLES 12. Which expression represents the number of atoms in 1.0 10–3 g of lead? 3 1 1 3 5 7 mass (g) 9 What is the density of the material? (A) 1.5 g·cm (B) 2.0 g·cm (C) 0.67 g·cm (D) 0.50 g·cm 5. (A) (B) (C) (D) 8. (A) (B) (C) (D) Which is a unit for expressing volume? mm g cm3 g·cm (A) 207 (22.4) (1 .0 10 -3 ) -3 (B) (22.4) (1 .0 10 ) 207 (C) 207 (6.02 10 ) (1 .0 10 3 ) 23 23 -3 (D) (6.02 10 ) (1.0 10 ) 207 23 3 (E) (6.02 10 ) (1.0 10 ) 207 6. The number 149,000,000 is usually written in scientific notation as (A) 0.149 109 (B) 149 x 106 (C) 1.49 x 108 (D) 1490 x 105 13. How many atoms are in one mole of hydrogen sulfide, H2S? (A) 34 6.02 1023 (B) 3 6.02 1023 (C) 3 (D) 34 7. Which measurement is the most uncertain? (A) 1.00 ± 0.01 cm (B) 2.00 ± 0.05 L (C) 10 ± 1 g (D) 200 ± 1 mL 14. A substance whose density is 4.00 g·cm–3 occupies a volume of 12.0 cm3. What is its mass? (A) 0.333 g (B) 8.00 g (C) 48.0 g (D) 4.00 g REVIEW SHEET 1ST SEMESTER HONORS CHEMISTRY 21. Which is STP? 15. How many moles of oxygen atoms are present in one mole of beryllium sulfate tetrahydrate, BeSO4·4H2O? (A) eight (B) five (C) four (D) two 16. Which statement best accounts for the fact that gases can be greatly compressed? (A) Molecules occupy space. (B) The collisions of molecules are elastic. (C) Molecules of gases are in constant motion. (D) The molecules of a given gas are identical. (E) Molecules of gases are relatively far from each other. 17. Gases may be most easily liquefied by (A) (B) (C) (D) (E) raising the temperature and lowering the pressure. raising the pressure and lowering the temperature. lowering both the temperature and pressure. raising both the temperature and pressure. lowering the temperature and keeping the presure unchanged. (A) (B) (C) (D) (E) 0 °C and 76 mmHg 0 K and 76 mmHg 0 K and 760 mmHg 100 °C and 76 cmHg 273 K and 760 mmHg 22. A student collects one liter samples of O2, CO2, and CH4 at laboratory conditions. What quantity is the same for all three samples? (A) number of atoms divided by the number of molecules in each sample (B) number of molecules in each sample (C) number of atoms in each sample (D) mass of each sample 23. Each of three identical containers holds a mole of gas, all at the same temperature. CH O SO 18. If the temperature and pressure are the same, one gram of hydrogen has about the same number of atoms as Atomic Molar Masses (A) (B) (C) (D) (E) H O 1 g of oxygen. 2 g of oxygen. 8 g of oxygen. 16 g of oxygen 32 g of oxygen 1.0 g·mol–1 16.0 g·mol–1 . . 19. One liter of oxygen at STP contains approximately the same number of molecules as (A) 2 L of He at STP. (B) 1/3 L of O3 at STP. (C) l L of CO2 at STP (D) 1/5 L of CH4 at STP. (E) (D) (E) 250 mL of NH3 at STP. 20. According to the Avogadro Principle, one liter of gaseous hydrogen and one liter of gaseous ammonia contain the same number of (A) (B) (C) (D) atoms at standard conditions. molecules at all conditions. molecules only at standard conditions. atoms if conditions in both containers are the same. (E) molecules if conditions in both containers are the same. Which gas exerts the greatest pressure? Assume ideal behavior. (A) (B) (C) (D) CH4 O2 SO2 They all exert the the same pressure. 24. A weather balloon contains 12 L of hydrogen at 740 mmHg pressure. At what pressure in mmHg will the volume become 20 L (temperature constant)? (A) (B) (C) (D) (E) 370 444 760 1230 1480 25. A gas occupies a volume of 2.0 cubic feet at 13 atm. How many cubic feet does this gas occupy at 1.0 atm, temperature constant? (A) (B) (C) (D) 6.5 13 15 26 REVIEW SHEET 1ST SEMESTER HONORS CHEMISTRY 26. A sample of gas at 1.00 atm of pressure occupies a volume of 500 L. If the volume is decreased to 125 L and the temperature is held constant, what is the new pressure in atmospheres? (A) (B) (C) (D) Atomic Molar Masses C H Mg O 0.250 2.00 1.25 4.00 27. For a given amount of dry gas at constant temperature, when the pressure is doubled the volume is (A) (B) (C) (D) 31. The molar mass of magnesium acetate, Mg(C2H3O2)2, in g·mol–1 is halved. unchanged. doubled. increased, but not doubled. (A) (B) (C) (D) (E) 15 16 83 142 166 32. How many mole(s) of calcium carbonate, CaCO3, is represented by 50 g of the compound? 28. Approximately how many molecules are in 11 g of carbon dioxide, CO2, gas? Atomic Molar Masses Ca C O Atomic Molar Masses C O (A) (B) (C) (D) 12.0 g·mol–1 16.0 g·mol–1 1.5 1023 3.0 1023 6.0 1023 2.4 1023 29. What is the mass of one mole of calcium nitrate, Ca(NO3)2? (A) (B) (C) (D) (E) (A) (B) (C) (D) 40. g·mol–1 14. g·mol–1 16. g·mol–1 82 g 102 g 164 g 204 g 30. The number of moles of water in 1,000 g of water is 40.1 g·mol–1 12.0 g·mol–1 16.0 g·mol–1 1.0 2.0 0.20 4.0 0.50 33. The molar mass of aluminum sulfate, Al2(SO4)3, is Atomic Molar Masses Al O S Atomic Molar Masses Ca N O 12. g·mol–1 1. g·mol–1 24. g·mol–1 16. g·mol–1 (A) (B) (C) (D) (E) 27 g·mol–1 16 g·mol–1 32 g·mol–1 150 g·mol–1 170 g·mol–1 278 g·mol–1 342 g·mol–1 450 g·mol–1 34. The mass of one mole of ammonium carbonate, (NH4)2CO3, is approximately Atomic Molar Masses H O (A) (B) (C) (D) (E) 18.0 55.5 180.0 1000.0 18,000.0 Atomic Molar Masses 1.0 g·mol–1 16.0 g·mol–1 C H N O (A) (B) (C) (D) 43.0 g 72.0 g 78.0 g 96.0 g 12.0 g·mol–1 1.0 g·mol–1 14.0 g·mol–1 16.0 g·mol–1 REVIEW SHEET 1ST SEMESTER HONORS CHEMISTRY 41. Which formula is incorrect? 35. The number of molecules present in 22.0 g of carbon dioxide at STP is Atomic Molar Masses C O (A) (B) (C) (D) (E) 12.0 g·mol–1 16.0 g·mol–1 (A) (B) (C) (D) (E) 2.01 1012 6.03 1012 2.06 1022 3.01 1023 6.02 1023 AlSO4 Al2SO4 Al3SO4 Al3(SO4)2 Al2(SO4)3 43. What is the formula for chromium(III) oxide? (A) CrO (B) Cr2O (C) Cr3O (D) Cr2O3 44. What is the formula for strontium sulfide? Atomic Molar Masses H N O (D) NH4HSO4 (E) LiH 42. What is the formula for aluminum sulfate? 36. What mass of nitrogen dioxide, NO2, has the same number of molecules as 18.0 g of water, H2O? (A) (B) (C) (D) (A) Al2(SO4)3 (B) BaHCO3 (C) Ca(OH)2 1.0 g·mol–1 14.0 g·mol–1 16.0 g·mol–1 (A) SrS (B) Sr2S (C) SrS2 (D) SrS3 45. What is the formula for copper(II) hydroxide? (A) CuOH 6.02 g 18.0 g 23.0 g 46.0 g (B) Cu(OH)2 (C) Cu2OH (D) CuOH2 46. Which is the formula for ammonium nitrate? 37. Calculate the mass of 12.0 chlorine gas, Cl2. 1023 molecules of (A) NH3N (B) NH4N (D) NH4NO3 (C) NH4NO2 47. What is the formula for sodium carbonate? Atomic Molar Mass Cl (A) NaHCO3 (B) NaCO3 35.5 g·mol–1 (A) 35.5 g (B) 71.0 g (C) 142 g (D) 284 g UNIT 3 NOMENCLATURE 38. The correct formula for iron(III) sulfate is (A) FeSO4 (B) Fe(SO4)2 (C) Fe2SO4 (D) Fe2(SO4)3 (E) Fe3(SO4)2 39. The one correct formula among these is (A) Na2OH (B) Cu(SO4)2 (C) ZnCl2 (D) Zn(NO3)3 (E) BaNO3 40. Which formula is incorrect? (A) BaHCO3 (B) Ca(OH)2 (C) Al2O3 (E) ZnCO3 (D) K2SO4 (C) So2CO3 (D) Na2CO3 48. What is the formula for chromium(III) sulfate? (A) Cr2(SO4)3 (B) Cr3(SO4)2 (C) Cr2(SO3)3 (D) Cr3SO4 49. Which formula is followed by its correct name? (A) (B) (C) (D) (E) FeCl3, iron(III) chloride FeS, iron(II) sulfite Mg3N2, magnesium nitrite KNO2, potassium nitrate HClO, hydrochloric oxide 50. The compound not properly named is (A) (B) (C) (D) (E) Fe2O3, iron(III) oxide. Pb3O4, lead(III) tetraoxide. CuCl2, copper(II) chloride. Pb3(PO4)2, lead(III) phosphate. P2S5, diphosphorus pentasulfide. REVIEW SHEET 1ST SEMESTER HONORS CHEMISTRY 51. What is the name of the compound having the formula CaH2? 61. The total number of atoms represented by 5Al(C2H3O2)3 is (A) calcium amide (B) calcium hydride (A) (B) (C) (D) (E) (C) calcium hydrate (D) calcium hydroxide 52. What is the name of the compound Fe2(SO4)3? (A) iron(II) sulfate (B) iron(III) sulfate (C) iron(II) trisulfate (D) iron(II) sulfate(III) 53. What is the correct name for Fe(NO3)2? (A) iron(II) nitrate (C) iron(III) nitrate (B) iron(II) nitrite (D) iron(III) nitrite 54. The formula for hydrogen bromate is HBrO3, and the formula for dysprosium oxide is Dy2O3. What is the formula for dysprosium bromate? (A) Dy2BrO3 (B) Dy3BrO3 (C) Dy(BrO3)3 (D) Dy2(BrO3)3 55. The formula for ytterbium sulfate is Yb2(SO4)3. What is the formula for ytterbium chloride? (A) YbCl2 (B) Yb2Cl3 (C) Yb2Cl2 (C) NO2–, NO3– (D) HS–, HSO4– 57. Barium perrhenate has this formula: Ba(ReO4)2. The perrhenate ion is (A) ReO4– (B) ReO42– (C) ReO43– (D) ReO44– 58. What is the total number of oxygen atoms represented by the formula KAl(SO4)2·12H2O? (A) 9 (B) 16 (C) 20 (D) 48 (E) 96 59. Which is the number of atoms of hydrogen in one molecule of glycerine, C3H5(OH)3? (A) 14 (B) 8 (C) 6 (D) 5 60. The total number of atoms represented by the formula K3Fe(CN)6 is (A) (B) (C) (D) (E) 4 10 11 16 36 62. The number of atoms of oxygen indicated by the formula Ca3(PO4)2 is (A) (B) (C) (D) (E) 12 8 7 4 3 63. How many atoms are in one molecule of acetone, CH3COCH3 ? (A) 1 (B) 6 (C) 3 (D) 10 64. Using only these formulas, XY2 (D) YbCl3 56. In which pair of anions do both names end in ‘–ate’? (A) Cl–, ClO3– (B) ClO3–, NO3– 22 60 71 84 110 X2 Z QZ what formula would you expect for a compound of elements Q and Y? (A) QY (B) QY2 (C) Q2Y (D) QY4 65. Which set consists only of compounds? (A) Na, Ca, He (B) H3O+, Cl–, I3– (C) NaCl, CH4, Br2 (D) H2S, CuCl2, KI 66. Which substance contains only one kind of atom? (A) water (C) aluminum (B) ethanol (D) carbon dioxide UNIT4 BALANCE EQUATIONS (REG, IONIC, NETIONIC STOICHIOMETRY AND LIMITNG REACTANTS 67. Which property is always conserved during a chemical reaction? (A) mass (B) volume (C) pressure (D) solubility 68. The equation Cu + 4HNO3 Cu(NO3)2 + 2H2O + ? would be completed and balanced by using (A) NO2 (E) 2NO (B) 2NO2 (C) 3NO2 (D) 4NO2 REVIEW SHEET 1ST SEMESTER HONORS CHEMISTRY 69. When the equation 75. Which set of coefficients correctly balances the equation? ? Sb + ? Cl2 ? SbCl3 ? Al(s) + ? H+(aq) ? Al3+(aq) + ? H2(g) is correctly balanced, the sum of the coefficients is (A) 2 (B) 3 (C) 6 (D) 7 (E) 9 (A) 1, 2, 1, 2 (B) 2, 6, 2, 3 (C) 3, 2, 3, 2 (D) 2, 3, 2, 3 76. Which equation represents the complete combustion of acetylene in an excess of air? 70. Which expression is correctly balanced? (A) (B) (C) (D) (E) (A) (B) (C) (D) (E) Na2O2 + 2H2O 2NaOH + O2 2Na2O2 + 2H2O 4NaOH + 2O2 4Na2O2 + 3H2O 4NaOH + 2O2 2Na2O2 + 2H2O 4NaOH + O2 3Na2O2 + 2H2O 6NaOH + O2 71. Which set of coefficients balances this equation? ? CH4(g) (A) 3, 1, 1 ? C3H8(g) + ? H2(g) (B) 3, 2, 1 (C) 3, 1, 2 C2H2 + 2O2 2CO2 + H2 C2H2 + O2 2CO + H2 C2H2 + O 2C + H2O C2H2 + O2 2C + H2O2 2C2H2 + 5O2 4CO2 + 2H2O 77. Dysprosium oxide, Dy2O3, reacts with hydrochloric acid to produce only water and a salt. The salt is (A) Dy2Cl3 (D) 6, 2, 2 (B) DyCl2 (C) DyCl3 (D) DyCl6 78. Which equation represents the dissolving of sodium sulfate, Na2SO4, in water? (E) 6, 2, 6 72. Consider the unbalanced expression: ? CH3CH2CHO(l) + ? O2(g) ? CO2(g) + ? H2O(g) Which set of coefficients balances the equation? (A) 2, 8, 3, 6 (B) 3, 8, 6, 6 (C) 1, 4, 3, 2 (D) 1, 8, 3, 3 (E) 1, 4, 3, 3 73. Consider the unbalanced expression: ? Cu(s) + ? NO3–(aq) + ? H+(aq) ? Cu2+(aq) + ? NO(g) + ? H2O(l) Which set of coefficients correctly balances the equation? (A) 4, 5, 3, 8, 2, 3 (B) 2, 4, 3, 8, 3, 3 (C) 3, 2, 8, 7, 2, 4 (D) 3, l, 8, 7, 4, 2 (E) 3, 2, 8, 3, 2, 4 74. The expression for pentane, C5H12, burning in oxygen is ? C5H12(g) + ? O2(g) ? CO2(g) + ? H2O(g) What set of coefficients balances the equation? (A) 1, 8, 5, 6 (B) 2, 8, 10, 6 (C) 1, 8, 5, 12 (D) 1, 11, 5, 12 (A) (B) (C) (D) Na2SO4(s) Na2+(aq) + SO42–(aq) Na2SO4(s) 2Na+(aq) + SO42–(aq) Na2SO4(s) Na22+(aq) + S2–(aq) + 4O2–(aq) Na2SO4(s) 2Na2+(aq) + S2–(aq) + O2–(aq) 79. What is the net ionic equation for the reaction between solutions of sodium chloride, NaCl, and silver nitrate, AgNO3? (A) (B) (C) (D) Na+(aq) + NO3–(aq) Na(s) + 1/2N2(g) + 3/2O2(g) Ag+(aq) + Cl–(aq) Ag(s) + 1/2Cl2(g) + – Ag+(aq) + Cl–(aq) Ag (aq) + Cl (aq) Ag+(aq) + Cl–(aq) AgCl(s) 80. Which equation represents the dissolving (dissociation) of aluminum sulfate, Al2(SO4)3, in water? (A) (B) (C) (D) Al2(SO4)3(s) 2Al3+(aq) + 3S6+(aq) + 4O2–(aq) Al2(SO4)3(s) 2Al3+(aq) + 3SO42–(aq) Al2(SO4)3(s) 2Al2+(aq) + 3SO43–(aq) Al2(SO4)3(s) Al3+(aq) + SO43–(aq) REVIEW SHEET 1ST SEMESTER HONORS CHEMISTRY 81. The overall equation for the reaction between KCl and AgNO3 is K+(aq) + Cl–(aq) + Ag+(aq) + NO3–(aq) K+(aq) + NO3–(aq) + AgCl(s) What is the net ionic equation? (A) (B) (C) (D) Ag+(aq) + Cl–(aq) AgCl(s) K+(aq) + Cl–(aq) KCl(s) K+(aq) + NO3–(aq) KNO3(s) K+(aq) + Cl–(aq) + Ag+(aq) + NO3–(aq) Ag+(aq) + K+(aq) + Cl–(aq) + NO3–(aq) 82. Complete the equation for the reaction between solutions of lead nitrate, Pb(NO3)2, and ammonium sulfide, (NH4)2S. Pb2+(aq) + 2NO3–(aq) + 2NH4+(aq) + S2–(aq) (A) 2NH4NO3(s) + Pb2+(aq) + S2–(aq) (B) Pb(NO3)2(s) + 2 NH4+(aq) + S2–(aq) (C) (NH4)2S(s) + Pb2+(aq) + 2NO3–(aq) (D) PbS(s) + 2NH4+(aq) + 2NO3–(aq) 83. Which is the balanced net ionic equation for the formation of the precipitate silver chromate, Ag2CrO4? (A) (B) (C) (D) 2Ag+(aq) + CrO42–(aq) Ag2CrO4(s) Ag+(aq) + CrO42–(aq) Ag2CrO4(s) Ag0(aq) + CrO42–(aq) Ag2CrO4(s) Ag2CrO4(s) 2Ag+(aq) + CrO42–(aq) 86. Which is the net ionic equation for the reaction of lead(II) nitrate and sodium chromate? (A) Pb2+(aq) + CrO42–(aq) PbCrO4(s) (B) Pb(NO3)2(aq) + Na2CrO4(aq) PbCrO4(s) + 2NaNO3(aq) (C) 2Na+(aq) + CrO42–(aq) Na2CrO4(aq) (D) Pb2+(aq) + NO3–(aq) + Na+(aq) + CrO42–(aq) PbCrO4(s) + Na+(aq) + NO3–(aq) 87. What is the net ionic equation for the reaction between lead(II) nitrate and potassium sulfide? (A) (B) (C) (D) Pb2+(aq) + S2–(aq) PbS(s) K+(aq) + NO3–(aq) KNO3(aq) Pb(NO3)2(aq) + K2S(aq) PbS(s) + 2KNO3(aq) Pb2+(aq) + 2NO3–(aq) + 2K+(aq) + S2–(aq) PbS(s) + 2K+(aq) + 2NO3–(aq) STOICHIOMETRY W/LIMITING REACTANTS 88. 50.0 g of water is heated from 22.0 °C to 36.0 °C. How much heat is absorbed? Specific Heat Capacity for Water 4.18 J·°C–1·g–1 (A) 1510 J (C) K+ and Cl– (D) Ag+ and Cl– (C) 4520 J (D) 4600 J (E) 7520 J 89. How much heat is required to raise the temperature of 25.0 g of iron from 10.0 °C to 40.0 °C? Specific Heat Capacity of Iron 84. Which two ions do not participate in the reaction between solutions of silver nitrate, AgNO3, and potassium chloride, KCl? (A) K+ and Ag+ (B) K+ and NO3– (B) 2930 J 0.444 J·g–1·°C–1 (A) 750 J (B) 444 J (C) 333 J (D) 313 J 90. What volume is occupied by 2.00 g of a substance having a density of 5.00 g·cm–3? 85. In the equation: BaCl2(aq) + Na2SO4(aq) BaSO4(s) +2NaCl (aq) What is the net ionic equation for this reaction? (A) (B) (C) (D) Cl–(aq) + Na+(aq) NaCl (aq) Cl22–(aq) + Na22+(aq) 2NaCl (aq) Ba2+(aq) + SO42–(aq) BaSO4(s) BaCl2(s) + Na2SO4(s) Ba2+(aq) + 2Cl–(aq) + 2Na+(aq) + SO42–(aq) (A) 0.400 cm3 (B) 2.50 cm3 (C) 7.00 cm3 (D) 10.0 cm3 91. If 50 mL of a 200 mL sample of 0.10 M sodium chloride solution is spilled, what is the concentration of the remaining solution? (A) 0.20 M (B) 0.10 M (C) 0.075 M (D) 0.025 M REVIEW SHEET 1ST SEMESTER HONORS CHEMISTRY 92. In the reaction 97. How many grams of calcium carbonate, CaCO3, would be needed to produce 44.8 L of carbon dioxide gas, CO2, measured at STP? 4Al + 3O2 2Al2O3 how many moles of aluminum oxide, Al2O3, are produced from one mole of aluminum, Al? (A) 0.5 (B) 2 (C) 3 Atomic Molar Masses (D) 4 93. Given the equation N2 + 3H2 CaCO3 + 2HCl CaCl2 + H2O + CO2 2NH3 (A) 50.0 Theoretically, the number of moles of ammonia produced from 2 mol of nitrogen is (A) 1 (B) 2 (C) 3 (D) 4 C4H4O4 + NaOH NaC4'H3O4 + H2O C4H4O4 + 2NaOH Na2C4H2O4 + 2H2O C4H4O4 + 3NaOH Na3C4HO4 + 3H2O C4H4O4 + 4NaOH Na4C4O4 + 4H2O 95. In neutralizing 0.015 mol of H3PO3, 0.030 mol of NaOH was consumed. Which equation describes this reaction? H3PO3 + NaOH NaPO3 + H2O H3PO3 + NaOH NaH2PO3 + H2O H3PO3 + 3NaOH NaPO3 + 3H2O H3PO3 + 2NaOH Na2HPO3 + 2H2O 96. Silica, SiO2, reacts with hydrofluoric acid, HF, according to this equation SiO2 + 4HF 2H2O + SiF4 Which reagent is completely consumed when 2 mol of SiO2 is added to 6 mol of HF? (A) SiF4 (C) 111 (D) 200 2KClO3 2KCl + 3O2 94. In an experiment, 0.0041 mol of maleic acid, C4H4O4, reacted with 0.0082 mol of sodium hydroxide, NaOH. Which equation describes the reaction? (A) (B) (C) (D) (B) 100 98. What volume of oxygen, O2, at STP can be prepared by the complete decomposition of 0.100 mol of potassium chlorate, KClO3? (E) 5 (A) (B) (C) (D) 40.1 g·mol–1 12.0 g·mol–1 16.0 g·mol–1 Ca C O (B) H2O (C) HF (D) SiO2 (A) 1.49 L (B) 3.36 L (C) 4.80 L (D) 6.72 L 99. The equilibrium equation for the Haber process at 500 °C is N2 + 3H2 2NH3 + heat When one liter of nitrogen combines with three liters of hydrogen the maximum volume of ammonia produced is (A) 1 L (B) 2 L (C) 3 L (D) 4 L (E) 6 L 100. The volume of pure oxygen needed to burn completely 800 mL of acetylene (C2H2) gas is (A) 800 mL (B) 1600 mL (C) 2000 mL (D) 10000 mL (E) 20000 mL 101. A mixture of 2.0 g of hydrogen and 32 g of oxygen is exploded and produces water. What mass of gas remains uncombined? Atomic Molar Masses H O (A) 1.0 g of hydrogen (B) 1.0 g of oxygen (C) 8.0 g of oxygen 1.0 g·mol–1 16.0 g·mol–1 (D) 16 g of oxygen (E) 24 g of oxygen REVIEW SHEET 1ST SEMESTER HONORS CHEMISTRY 102. The equation for the complete combustion of butane gas, C4H10, is 2C4H10 + 13O2 8CO2 + 10H2O How many liters of carbon dioxide is produced when a mixture of 1.00 L of butane gas and 13.0 L of oxygen is burned? (measured under the same conditions) (A) 1.00 L (B) l. 63 L (C) 8.00 L 106. In the reaction represented by the equation COCl2 + 2NaI 2NaCl + CO + I2 what is the maximum mass of iodine that can be liberated from 60.0 g of sodium iodide? Molar Masses 150. g·mol–1 254. g·mol–1 NaI I2 (D) 4.00 L (E) 13.0 L (A) 5.00 g 103. The mass of potassium chloride formed by the complete decomposition of 490 g of potassium chlorate is (A) 96 g 35.5 g·mol–1 39.1 g·mol–1 16.0 g·mol–1 (B) 122.5 g (C) 149 g (C) 50.8 g (D) 127 g (E) 254 g 107. What mass of iron oxide, Fe3O4, is produced from 2.00 mol of iron, Fe? Atomic Molar Masses Cl K O (B) 25.4 g 3Fe(s) + 4H2O(g) Fe3O4(s) + 4H2(g) Molar Mass Fe3O4 (D) 298 g 231. g·mol–1 (E) 490 g (A) 154 g 104. In the reaction 108. What mass of calcium hydroxide, Ca(OH)2, is obtained from 18.7 g of calcium oxide, CaO? 2Al + 3H2SO4 3H2 + Al2(SO4)3 (B) 9.0 g (C) 13.5 g Ca H O (D) 27.0 g (E) 81.0 g (D) 693 g 40.1 g·mol–1 1.0 g·mol–1 16.0 g·mol–1 CaO + H2O Ca(OH)2 105. What is the maximum mass of tungsten (W) obtained from the use of 18 g of hydrogen according to the equation (A) 18.7 g Atomic Molar Masses H W 1. g·mol–1 184. g·mol–1 (B) 24.7 g (C) 56.1 g (D) 74.1 g 109. Consider the equation: WO3 + 3H2 W + 3H2O (A) 1 184 g (B) 3 184 g (C) 9 184 g (C) 462 g Atomic Molar Masses the mass of aluminum that reacts with 1 mol of hydrogen ions is approximately (A) 3.0 g (B) 231 g 2Al(OH)3 Al2O3 + 3H2O When 15.0 g of aluminum hydroxide, Al(OH) 3 is decomposed, how many grams of water will be formed? Atomic Molar Masses (D) 18 184 g (E) 184 g + 3 16 g Al H O (A) 3.86 g (B) 5.19 g 27.0 g·mol–1 1.0 g·mol–1 16.0 g·mol–1 (C) 4.20 g (D) 22.5 g REVIEW SHEET 1ST SEMESTER HONORS CHEMISTRY 110. What mass of water is produced by complete combustion of 126 g of propene, C3H6? 2C3H6 + 9O2 6H2O + 6CO2 114. How many grams of water, H2O, can be prepared when 2.00 mol of hydrogen, H2, and 2.00 mol of oxygen, O2, are mixed and reacted in this process? 2H2 + O2 2H2O Atomic Molar Masses 12.0 g·mol–1 1.0 g·mol–1 16.0 g·mol–1 C H O (A) 18.0 g (B) 54.0 g (C) 126 g Atomic Molar Masses H O (D) 162 g 111. If 10.0 g of iron, Fe, and 10.0 g of sulfur, S, are heated together, how many grams of iron(II) sulfide, FeS, could be formed? Atomic Molar Masses (A) 18.0 g (B) 36.0 g (B) 15.7 (C) 27.6 Atomic Molar Masses B Na O C3H8(g) + 5O2(g) 3CO2(g) + 4H2(g) (A) Na2B4O7 (B) NaBO (C) NaB2O5 Atomic Molar Masses C H Atomic Molar Masses (B) 29.3 g 12.0 g·mol–1 16.0 g·mol–1 (C) 44.0 g (A) CH2 (E) C2H4 (D) 66.0 g 113. Consider the equation: (C) C2H (D) C2H2 Atomic Molar Masses C H How many moles of reactant are in excess when 2.0 mol of CH4(g) are ignited in 2.0 mol of O2(g)? (C) 0.5 mol CH4 (D) no excess of either reactant (B) CH 12 g·mol–1 1 g·mol–1 117. Decomposition of 12 g of a compound containing only carbon and hydrogen yields 9 g of carbon and 3 g of hydrogen. What is the simplest formula of the compound? CH4(g) + 2O2(g) CO2(g) + 2H2O(l) (A) l.0 mol CH4 (B) 2.0 mol O2 (D) Na3B4O (E) Na3BO4 116. A compound contains 85.71% carbon and 14.29% hydrogen by mass. Its simplest formula is What is the maximum mass of carbon dioxide produced when a mixture of 0.500 mol of propane and 3.00 mol of oxygen is ignited? (A) 22.0 g (E) 132. g 11 g·mol–1 23 g·mol–1 16 g·mol–1 (D) 88.0 112. The equation for the complete combustion of propane, C3H8, is C O (D) 72.0 g 115. Upon analysis a compound is found to contain 22.8% sodium, 21.8% boron, and 55.4% oxygen. Its simplest formula is Fe + S FeS (A) 10.0 (C) 68.0 g EMPIRICAL FORMULAS 55.8 g·mol–1 32.1 g·mol–1 Fe S 1.0 g·mol–1 16.0 g·mol–1 (A) CH2 (E) C3H9 (B) CH4 12.0 g·mol–1 1.0 g·mol–1 (C) C2H5 (D) C3H7 REVIEW SHEET 1ST SEMESTER HONORS CHEMISTRY 118. A sample of a compound contains 3.21 g of sulfur, S, and 11.4 g of fluorine, F. Find the empirical formula of the compound. Atomic Molar Masses F S (A) SF (B) SF2 19.0 g·mol–1 32.0 g·mol–1 (C) SF3 (D) SF6 (C) C12H4O2 (D) C12H24O12 120. A substance has an empirical (simplest) formula of CH3 and a molar mass of 30 g·mol–1. The molecular (true) formula is Atomic Molar Masses C H (A) an ion. (B) a radical. (C) an isotope. (A) losing protons. (B) losing electrons. (B) (CH3)2 52 3+ 24Cr ? (A) 52 (B) 27 (C) 24 (D) 21 127. The number of neutrons in the nucleus of an atom of 9 4Be is (A) 36 (E) 4 (B) 13 (C) 9 (D) 5 128. Which symbol represents an atom that contains the largest number of neutrons? g·mol–1 12.0 1.0 g·mol–1 (C) (CH3)3 (C) gaining protons. (D) gaining electrons. 126. How many electrons are in a chromium(III) ion, 235 (A) 92U (A) (CH3)1 (D) a molecule. (E) an electrolyte. 125. Metallic atoms become ions by 119. A compound has the empirical formula CH2O and the molecular mass 180 g·mol–1. What is its molecular formula? (A) CH8O10 (B) C6H12O6 124. An atom that loses or gains an electron becomes 239 (B) 92U 239 (C) 93Np 239 (D) 94Pu (D) (CH3)4 231 121. A compound whose empirical formula is CH2 has a molar mass of 28 g·mol–1. What is the molecular formula? Atomic Molar Masses C H (A) CH2 (B) C2H4 129. An ion has 13 electrons, 12 protons, and 14 neutrons. What is the mass of the ion? (A) 14 u (E) 39 u 12.0 g·mol–1 1.0 g·mol–1 (C) C2H2 (E) 91Pa (D) CH4 F S (A) SF (B) S2F2 19. g·mol–1 32. g·mol–1 (C) S3F3 (D) SF4 UNIT 7 atomic theory 9-10 PERIOD TABLE/TRENDS 123. A calcium ion is a calcium atom that has (A) lost one electron. (B) gained one electron. (C) gained one ion. (A) 11Na+ (D) lost two electrons. (E) gained two electrons. (D) 27 u 23 (B) 11Na 23 23 (C) 12Mg2+ (D) 12Mg 131. The atomic number of an element is determined by the number of (A) (B) (C) (D) (E) Atomic Molar Masses (C) 26 u 130. The symbol that represents 11 protons, 12 neutrons, and 10 electrons would be: 23 122. A gaseous compound contains a ratio of one atom of sulfur to one atom of fluorine. A mole of this gas has a mass of approximately 102 g. What is the molecular formula? (B) 25 u protons in each of its atoms. neutrons in each of its atoms. particles in each of its atoms. protons plus neutrons in each of its atoms. protons plus electrons in each of its atoms. 132. All positive ions differ from their corresponding atoms by having (A) (B) (C) (D) (E) larger diameters. fewer electrons. a charge of +1. greater atomic masses. stronger metallic properties REVIEW SHEET 1ST SEMESTER HONORS CHEMISTRY 133. Which group represents particles that contain the same number of electrons? (D) O2–, S2–, Se2– (E) Ca2+, Fe2+, Zn2+ (A) F, Ne, Na (B) Mg, Al, Si (C) Cl–, Ar, K+ 134. Note the chart of interactions of equal volumes of various 0.100 M aqueous solutions. (Symbols of elements or ions have been replaced by capital letters, and soluble products are indicated by “S”) What is the formula of the precipitate? 139. In the modern periodic table the elements are arranged in the order of increasing (A) atomic masses. (B) atomic radii. (C) atomic numbers. (D) atomic volumes. 140. In which set are the three elements in the same family? (A) B, C, N (B) N, O, F (C) Hg, Ga, Sr (D) Zn, Cd, Hg 141. Which scientist is given credit for developing the periodic table? AY BX CY D X pp t S CY S S B X pp t (A) DY (B) BY (A) Rutherford (B) Mendeleev S 142. lf XO2 is the correct formula for an oxide, the formula for the chloride of X is (C) AX (D) CX 135. An odorless, colorless, tasteless gas is suspected to be oxygen. Which result would support this hypothesis? (A) (B) (C) (D) The gas would extinguish a flame. The gas would turn limewater milky. The gas would burn in air producing only water. A glowing splint would burst into flame in the gas. 136. The chemical properties of atoms depend principally upon (A) (B) (C) (D) their atomic masses. the masses of the nuclei involved. the number of neutrons in their nuclei. the ratio in which the atoms combine with other atoms. (E) the number of electrons in their outermost shells. 137. The similar chemical behavior of the elements in a given family in the periodic table is best accounted for by the fact that atoms of these elements have (A) (B) (C) (D) (E) the same number of electrons in the outermost shell. the same number of electrons. the same number of protons. similar nuclear structures. a common origin 138. The best explanation of the extreme activity of fluorine as compared to other halogens is that the fluorine atom (A) (B) (C) (D) (E) (C) Dalton (D) Planck has the smallest atomic radius. has the smallest nuclear charge. has seven valence electrons. is the strongest reducing agent. needs one electron to complete its outermost shell. (A) XCl2 (B) XCl4 (C) XCl (D) X2Cl3 (E) XCl3 143. M represents a metallic element, the oxide of which has the formula M2O. The formula of the chloride of M is (A) MCl (B) MCl2 (C) MCl3 (D) MCl4 (E) M2Cl 144. What is the most probable formula for a compound of silicon, Si, and hydrogen, H? (A) SiH (B) SiH2 (C) SiH6 (D) SiH4 145. A hypothetical element, Z, forms a chloride with the formula ZCl5. What is the most probable formula for its oxide? (A) ZO2 (B) ZO5 (C) Z2O5 (D) Z5O2 146. Based on the position of the elements in the periodic chart, the most likely formula for strontium nitride is (A) Sr2N5 (B) Sr5N2 (C) Sr2N3 (D) Sr3N2 147. Which family of elements always forms ions with an oxidation number of +2 in compounds? (A) halogens (B) alkali metals (C) transition metals (D) alkaline–earth metals 148. Which element is the most electronegative? (A) Be (B) Mg (C) Ca (D) Sr (E) Ba REVIEW SHEET 1ST SEMESTER HONORS CHEMISTRY 149. Since sodium and potassium are both members of Group 1A in the periodic table, a sodium and a potassium atom have the same 152. Consider a plot of a property of the alkaline earth metals. (A) (B) (C) (D) atomic mass. number of protons in their nuclei. atomic number and the same nuclear charge. characteristic of losing one electron per atom to form an ion. (E) total number of electrons around the nucleus. 150. The element requiring the least amount of energy to remove one electron from an atom is H Li Na Be Mg (A) Na B Al (B) Be C Si N P O S (C) O F Cl (D) Cl (E) Ar 151. In which part of the periodic table are the most electronegative elements found? He Ne Ar 4 12 20 38 Be Mg Ca Sr Atomic number 56 Ba Which property is plotted on this graph? (A) (B) (C) (D) first ionization energy atomic radius atomic mass number of valence electrons 153. As the atomic numbers of the elements in a family increase, the (A) (C) (B) (D ) (A) (B) (C) (D) (E) atomic radii decrease. atomic masses decrease. ionization energies decrease. elements become less metallic. number of electrons in the outermost energy level increases. 154. Which of these atoms has the smallest radius? (A) K (A) upper left (B) lower left (C) upper right (D) lower right (B) Cl (C) Br (D) Cs 155. Which characteristic of fluorine causes it to be the most active member of the halogen family, Group 7A? (A) (B) (C) (D) It forms diatomic molecules. It has the smallest atomic radius. It has no naturally occuring isotopes. It has seven electrons in its outer shell. UNIT 11-13 ATOMIC STRUCTURE/DIAGRAMMING ELECTRONS 156. The chemical activity of an atom is most closely related to the number and arrangement of its (A) protons. (B) neutrons. (C) isotopes. (D) electrons. REVIEW SHEET 1ST SEMESTER HONORS CHEMISTRY 157. The molar mass of a compound is 75 g·mol–1. A student reported an experimental value of 78 g·mol–1. The percent error is 164. Which element has the electron configuration 1s22s22p63s23p6 4s13d5? (A) 78 – 75 78 (D) 78 – 75 100 75 (A) zinc (B) copper (C) nickel (B) 75 100 78 (E) 78 – 75 100 78 165. The electron arrangement that represents the most active metallic element in this list is (C) 78 100 75 (A) 2)7 (B) 2)8)1 (C) 2)8)2 (D) 2)8)3 (E) 2)8)6 158. A student reads a balance as 38.81 g. The correct reading is 38.41 g. What is the percent error? (A) 0.0104% (B) 0.104% (C) 0.400% (D) 1.04% (B) 50 (C) 51 166. What is the electronic configuration of an aluminum 27 atom, 13Al? 159. The number of protons in the atom whose atomic mass is 89 and atomic number is 39, is (A) 39 (D) chromium (E) potassium (D) 89 (A) (B) (C) (D) (E) ls22s22p63d3 1s22s22p63s23p1 ls22s22p62d13s2 1s22s22p62d103s23p5 1s22s22p63s23p63d74s2 (E) 128 167. Which atom contains a partially filled 3p orbital? 160. The particles present in the orbitals of an atom are (A) iron (B) argon (C) boron (A) mesons. (B) protons. (C) neutrons. (D) positrons. (E) electrons. 161. A neutral atom whose outermost electron shell contains eight electrons (A) (B) (C) (D) (E) is very active. has a combining number of one is classified as a metal. is chemically inert. is more active than hydrogen. 162. When the halogens form ions, the result is (A) (B) (C) (D) (E) colored ions. positive ions. diatomic molecules. covalent compounds. a completed outer shell of electrons. 163. The correct electronic configuration for the sodium 23 atom, 11Na, is (A) (B) (C) (D) (E) 1s22s22p6 1s22s22p63s1 1s22s22p43s23p1 1s22s22p82d103s1 1s22s22p62d103s23p1 (D) calcium (E) aluminum 168. Which element has the electron configuration 1s22s22p63s2? (A) aluminum (B) calcium (C) magnesium (D) sodium 169. Which electron configuration represents an atom in an excited state? (A) (B) (C) (D) 1s22s22p6 1s22s22p63s2 1s22s22p63s23p64s23d1 1s22s22p63s23p64s24p1 170. When two electrons occupy the same orbital, they must have (A) (B) (C) (D) (E) opposite spins. mutual attraction. four identical quantum numbers. different magnetic quantum numbers. different principal quantum numbers. 171. What is the maximum number of electrons allowed in an orbital? (A) 1 (E) 10 (B) 2 (C) 3 (D) 6 REVIEW SHEET 1ST SEMESTER HONORS CHEMISTRY 172. What neutral atom has the electron configuration 1s22s22p63s23p64s1? 178. Which electron configuration represents a transition element? (A) Na (A) (B) (C) (D) (B) K (C) Ca (D) Ba 173. Which sublevel becomes filled when a chloride ion, Cl–, is formed? (A) 2p (B) 3p (C) 4p (D) 3s 174. Which electron configuration represents a noble gas? (A) ls22s22p63s23p5 (B) ls22s22p63s23p6 (C) 1s22s22p63s23p64s1 (D) ls22s22p63s23p64s2 175. When an electron shifts from one energy level to a higher level in the same atom, energy is absorbed. Which of the electron transitions represented below absorbs (that is, requires) the most energy? BONDING 179. In which pair do both compounds exhibit ionic bonding? (A) SO2, HCl (B) KNO3, CH4 (C) NaF, KBr (D) KCl, CO2 (E) NaCl, H2O 180. A chemical bond is considered to be predominantly ionic if (A) atoms of the same element combine. (B) the reaction forming the bond is endothermic. (C) atoms of an active metal combine with the atoms of an active nonmetal. (D) the bond is between atoms of elements which are of the same family. (E) atoms of one metal combine with atoms of another metal. 4 3 A 1s22s22p63s2 1s22s22p63s23p6 1s22s22p63s23p64s1 1s22s22p63s23p63d3 4s2 D 2 181. Which bond has the least ionic character? B C (A) P—Cl (B) H—Cl (C) Br—Cl (D) S—Cl (E) Cl—Cl 1 (A) A (B) B 182. Which type of bonding predominates in solid potassium chloride, KCl? (C) C (D) D 176. A single burst of light is released from an atom. Which statement explains what happens in the atom? (A) An electron is changed from a particle to a wave. (B) An electron moved from a higher to a lower energy level. (C) An electron pulled a proton out of the nucleus. (D) An electron pulled a neutron out of the nucleus. 177. Neon atoms produce characteristic spectral lines when their electrons (A) (B) (C) (D) return to lower energy levels. orbit the nucleus in a single energy level. remain in their normal energy levels and move faster. remain in their normal energy levels and move slower. (A) ionic (B) metallic (C) hydrogen (D) covalent (molecular) 183. Which pair of elements react to form a compound that has the greatest ionic character? (A) xenon and fluorine (B) carbon and oxygen (C) cesium and chlorine (D) iron and sulfur 184. Which compound contains both ionic and covalent bonds? (A) CO2 (B) KNO3 (C) NaCl (D) CCl2F2 185. The electronegativity of francium is 0.7 and that of fluorine is 4.0. The difference in electronegativity suggests that the predominant bonding between Fr and F is (A) ionic. (B) metallic. (C) covalent. (D) very weak. (E) coordinate covalent REVIEW SHEET 1ST SEMESTER HONORS CHEMISTRY 186. A solid has no electrical conductivity at room temperature. It is heated to 600 °C, melts, and then has electrical conductivity. The solid has which type of bonding? (A) ionic bonding (B) covalent bonding 194. The graph below shows the boiling points of four hydrogen compounds. 120 100 80 60 40 20 0 -20 -40 -60 -80 (C) metallic bonding (D) van der Waals forces 187. The type of bond formed when two atoms share a pair of electrons is called (A) ionic. (B) double. (C) covalent (D) bivalent. (E) electrovalent. 188. A pure substance melts at 113 °C and does not conduct electricity in either the solid or liquid state. The bonding in this substance is primarily (A) ionic. (B) network. (A) Li and Br (B) Na and Br (C) K and Br (D) H and Br 190. When a chlorine molecule, Cl2, is formed, the orbital overlap may be represented by the designation (B) s – p (C) s – s (D) s – d (E) p – d POLARITY OF MOLECULES 191. Which represents a polar molecule? (A) F2 H Te HS 0 (C) metallic. (D) covalent (molecular). 189. Which pair of atoms forms a covalent bond? (A) p – p HO (B) O2 (C) CH4 (D) CO2 20 H Se 40 60 80 100 120 140 Molar Mass, g mol What type of bonding explains the large difference between the boiling points of H2O and the other hydrogen compounds? (A) ionic bonding (B) covalent bonding (C) hydrogen bonding (D) van der Waals attractions 195. An explanation of the heat of vaporization of water being much higher than the heat of vaporization of ethane (C2H6) is that (A) (B) (C) (D) (E) ethane has dipolar molecules. water is more dense than liquid ethane. water has a higher boiling point than ethane. water molecules are lighter than ethane molecules. energy is needed to break the hydrogen bonding between water molecules. (E) HCl 192. Which molecule is essentially nonpolar? (A) CH4 (B) HCl (C) HBr (D) H2O (E) NH3 193. The compounds H2S, H2Se, and H2Te boil below 0 °C at standard pressure. Water (H2O) boils at 100 °C. This abnormally high boiling point of water is a consequence of the (A) (B) (C) (D) (E) low molar mass of water. low electrical conductivity of water. covalent bonds in the water molecule. stability of the bonds in the water molecules. hydrogen bonds between the water molecules 196. The higher boiling point of HF compared with HCl, HBr, and HI is caused by (A) (B) (C) (D) (E) covalently bonded molecules. the size of the molecules. the shape of the molecules. hydrogen bonding between molecules. weak van der Waals forces between HF molecules. MOLECULAR SHAPES 197. Compounds that have the same molecular formula but different structural formulas are known as (A) isomers. (B) polymers. (C) isotopes. (D) allotropes. REVIEW SHEET 1ST SEMESTER HONORS CHEMISTRY 198. A molecule is said to be polar if it (A) (B) (C) (D) (E) 209. Which term best describes the shape of the ammonia, NH3, molecule? has a north and south pole. has a symmetrical electron distribution. exhibits a polar spin under certain conditions. may exhibit a positive or negative charge. exhibits a partial positive charge at one end and a partial negative charge at the other. (D) H–H Cl (B) O=C=O (E) Cl (C) tetrahedral (D) trigonal planar UNIT 8 RADIOACTIVITY/ATOMIC STRUCTURE 210. Rutherford’s alpha–particle bombardment of gold foil helped develop our current model of the atom by 199. Which represents a polar molecule? (A) H–Cl (A) linear (B) pyramidal C Cl (A) (B) (C) (D) Cl finding the mass of the electron. showing the existence of the neutron. showing that the electron carries a negative charge. showing that the atom has a concentrated central charge (C) NN 65 200. Which formula represents a nonpolar molecule? (A) HCl (B) CF4 (C) NH3 (D) H2S 201. Which is an example of a nonpolar molecule that contains polar covalent bonds? (A) CCl4 (B) N2 (C) H2O (D) NH3 202. Which molecule is nonpolar? (A) H2O (B) HF (C) NF3 (D) CF4 203. The shape of a chloroform molecule, CHCl3, is (A) linear. (B) cubical. (C) octahedral. (D) tetrahedral. (E) planar triangular. (B) HF (C) NF3 (A) (B) (C) (D) (E) 30 protons and 35 neutrons. 35 protons and 30 neutrons. 35 protons and 35 neutrons. 65 protons and 30 neutrons. 95 protons and 30 electrons. 212. Isotopes differ in (A) atomic number. (B) nuclear charge. (C) number of protons (D) CF4 205. The arrangement of atoms in a water molecule, H2O, is best described as (D) number of neutrons. (E) number of electrons. 213. A hypothetical element X has three isotopes: 40X, 41X, and 42X. Their abundances are 72.0%, 9.00%, and 19.0% respectively. What is the atomic mass of X? (A) 40.5 u 204. Which molecule is nonpolar? (A) H2O 211. The symbol 30Zn indicates this isotope contains (B) 40.8 u (C) 41.0 u (D) 41.5 u 214. Copper has an atomic molar mass of 63.5 g·mol–1. Why is the atomic molar mass not a whole number? 206. What is the shape of the ammonia, NH3, molecule? (A) All copper atoms have identical chemical properties. (B) The fractional number results from the fact that protons and neutrons have different masses. (C) There are at least two naturally occurring isotopes of copper. (D) Every copper atom has an atomic mass of 63.5 u. (A) bent (B) linear 215. The difference between the atomic number of an atom and its mass number gives the number of (A) ring. (B) bent. (C) linear. (D) spherical. (C) planar (D) pyramidal 207. The shape of the CH4 molecule is most similar to the shape of a molecule of (A) H2O (B) N2H4 (C) SiH4 (D) C2H4 208. Which molecule has all of its atoms in one plane? (A) H2SO4 (B) CH4 (C) BF3 (D) NH3 (A) protons. (B) neutrons. (C) energy levels. (D) orhitals. (E) electrons. REVIEW SHEET 1ST SEMESTER HONORS CHEMISTRY 216. Two kinds of emission from radioactive substances that are considered to be particles of matter are 222. Given the nuclear reaction 234 234 90Th 91Pa (A) alpha and beta emission. (B) alpha and gamma emission. +X What is X? 1 0 (C) beta and gamma emission. (D) gamma emission and X–radiation. (E) alpha emission and X–radiation. (A) A proton, 1p 217. What type of reaction is illustrated by this equation? 223. The half–life of radium is 1600 years. If a given sample contains one gram of radium, how much radium remains after 4800 years? 1 1H 3 1 4 (A) l g (C) a fission reaction (D) a fusion reaction 218. A radioactive element having atomic number 82 and 0 atomic mass 214 loses a beta particle, –1. The resulting element has Atomic No. Atomic Mass 80 81 81 82 83 210 u 213 u 214 u 213 u 214 u (A) (B) (C) (D) (E) (E) 234 91Pa (B) 236 92Th 234 92U (C) (D) (A) beta 1 234 90Th (D) 1/8 g 224. Strontium–90 has a half–life of 28 years. What fraction of a sample remains as strontium–90 after 84 years? (A) (B) (C) (D) (E) (B) 1/8 (C) 1/4 (D) 1/3 pressure of the air at 0 °C only. mass of a column of mercury. temperature of the air at standard pressure. density of mercury. pressure of the air. 226. Which apparatus delivers 50.00 mL of liquid most accurately? (A) (B) (C) (D) 50–mL buret 50–mL beaker 50–mL test tube 50–mL graduated cylinder 13 + 0n 6C + ? (B) alpha (C) 1/3 g 225. A barometer is used to measure the 220. Which particle completes the equation? 16 8O (B) 1/2 g LAB TECHNIQUES/PROCEDURES 238 238 92U (D) A beta particle, –1e (E) 1/16 g (A) 1/28 219. If the radioactive atom 92U emits an alpha particle, the atom remaining is represented by (A) 0 (B) A neutron, 0n + 1H 2He + energy (A) a chemical reaction (B) radioactive decay (C) A positron, +1e (C) proton (D) neutron 227. Most student thermometers have an uncertainty of 0.2 Celsius degrees. Which is the proper reading of the thermometer shown in the illustration? (E) deuteron 221. Which nuclide is produced when a radioactive carbon–14 atom emits an electron? 14 6C 14 (A) 6C (B) 14 7 17 16 15 14 0 ? + –1e N 13 (C) 6C 13 (D) 5B (A) l6. °C (B) 16.4 °C(C) 16.40 °C (D) 16.45 °C REVIEW SHEET 1ST SEMESTER HONORS CHEMISTRY 228. A narrow–necked, glass–stoppered bottle contains sulfuric acid. When the acid is being poured, the stopper should be (A) (B) (C) (D) placed on the lab table. put into the reaction vessel. held in the palm of the hand. held inverted between the index and middle fingers. 229. Which device is commonly used to measure liquid volumes most precisely? (A) graduated cylinder (B) graduated beaker (C) balance (D) buret 230. This drawing shows the surface of water in a 10 mL graduated cylinder. How much water is in the cylinder? 8 7 6 (A) 6.20 mL (B) 6.25 mL (C) 6.40 mL (D) 7.80 mL 231. Which device should be used to measure 22.5 mL of an aqueous solution? A (A) A B (B) B C (C) C D (D) D 232. In the laboratory, never dip a stirring rod into a reagent bottle because (A) (B) (C) (D) (E) the bottle may tip. the rod might break. the rod may puncture the bottle. the contents of the bottle may become contaminated. the amount of liquid remaining on the rod is too small to be used. 233. The purpose of filtration is to (A) (B) (C) (D) form precipitates. remove water from solutions. separate dissolved ions from the solvent. separate insoluble substances from a solution.