Preparation of 2-Hydroxy-3-Phenylpropanoic Acid Background In
advertisement

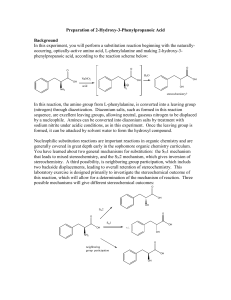
Preparation of 2-Hydroxy-3-Phenylpropanoic Acid Background In this experiment, you will perform a substitution reaction beginning with the naturallyoccurring, optically-active amino acid, L-phenylalanine and making 2-hydroxy-3phenylpropanoic acid, according to the reaction scheme below: O O O H2O NaNO2 OH OH OH acid OH N2 NH2 stereochemistry? In this laboratory experiment, the amino group from a naturally-occurring amino acid, phenylalanine, is converted into a leaving group (nitrogen) through diazotization. Diazonium salts, such as formed in this reaction sequence, are excellent leaving groups, allowing neutral, gaseous nitrogen to be displaced by a nucleophile. Amines can be converted into diazonium salts by treatment with sodium nitrite under acidic conditions, as in this experiment. Once the leaving group is formed, it can be attacked by solvent water to form the hydroxyl compound. Nucleophilic substitution reactions are important reactions in organic chemistry and are generally covered in great depth early in the sophomore organic chemistry curriculum. You have learned about two general mechanisms for substitution: the SN1 mechanism that leads to mixed stereochemistry, and the SN2 mechanism, which gives inversion of stereochemistry. A third possibility, is neighboring group participation, which includs two backside displacements, leading to overall retention of stereochemistry. This laboratory exercise is designed primarily to investigate the stereochemical outcome of this reaction, which will allow for a determination of the mechanism of reaction. Three possible mechanisms will give different stereochemical outcomes: O OH OH SN2 O O SN1 OH OH +/OH NH2 O neighboring group participation OH OH Verification of the stereochemical outcome of the reaction is readily available by measuring the optical rotation of the product and comparing it to the known literature value. Procedure: Week 1. To a 25-mL Erlenmeyer flask containing 1.65 g (10 mmol) L-phenylalanine and a magnetic stir bar, add 10 mL of 1M H2SO4 (caution: corrosive). Magnetically stir the solution at room temperature until homogeneous. Cool the solution to 3-5˚C in an icewater bath. Monitor the temperature with a thermometer clamped in place such that the magnetic stir bar does not hit the thermometer. Once the solution is cool, add 5 mL of a 3.0 M NaNO2 (caution: strong oxidizing agent) solution dropwise via a disposable pipette. The rate of the addition should be slow enough to maintain the reaction temperature below 5 ˚C and minimize the formation of noxious brown nitrogen oxide fumes. If a significant amount of brown fumes form, slow down the rate of addition. The addition should take about 45 minutes. During the addition, note bubbles of N2 gas forming. Once the addition is complete, remove the ice-water bath and allow the reaction to stir at room temperature until the end of the lab period. Lightly cork the Erlenmeyer to allow gas to escape, and leave the reaction in your drawer until the next lab period. Week 2. Cool the solution to 0-5 ˚C to maximize yield, and isolate the solid product by vacuum filtration. Wash the crystals by slurrying in 5 mL of ice-cold water in the Büchner funnel with the vacuum released. Remove the wash solvent by vacuum filtration. Pull air through the crystals until they are mainly dry. Spread out the crystals on a watch glass to dry further. Analyze the product by melting point (120-121.1 ˚C), 1H NMR (acetone-d6), and specific rotation (c = 1.4, acetone). Report Guidelines: Include typical results and observations as you have been learning for a synthetic lab. For the discussion, include comments on the success of the reaction based on experimental data. Why is the starting L-phenylalanine soluble in the reaction mixture but the product is not? Discuss the similarities and differences between the two structures in your answer. How high yielding was the reaction? How could it be improved? Did you form the product that you intended to form? (Does the proton NMR support this product—list all coupling constants and explain the splitting.) What is the stereochemistry of the product, and how is this supported? What is the enantiomeric excess of the reaction? Propose a mechanism for its formation. Explain how other mechanisms are excluded based on data.