Final-exam-review-fall
advertisement

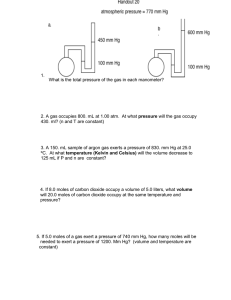
Chemistry Final Review 1. Complete and balance the following equation. Cd(NO ) NH Cl 2. Balance the following equation. NaClO NaCl O 3. Balance the following equation. Mg H PO Mg (PO ) H 4. Find the mass in grams of 3.10 10 molecules of F . 5. Find the number of moles of argon in 607 g of argon. 6. Find the mass, in grams, of 1.40 10 molecules of N . 7. Find the formula of the compound with the percent composition shown. 8. Compare and contrast an empirical formula and a molecular formula. 9. Show the correct dimensional analysis setup to convert 127.34 grams of LiNO3 to number of molecules, and find the final answer. 10. Label the diagram with the changes in state of matter that occur between each state. 11. Define the law that describes the partial pressures of gases present in a mixture. 12. An oxygen canister in a climbing camp high on Mount K-2 has a pressure of 5.00 atm at 25°C. If the temperature falls to -10°C, what is the new pressure inside the canister? 13. A gas at 110 kPa and 30.0°C fills a flexible container with an initial volume of 2.00 L. If the temperature is raised to 80.0°C and the pressure increases to 440 kPa, what is the new volume? 14. A helium balloon in a closed car occupies a volume of 2.32 L at 40.0°C. If the car is parked in the sun on a hot day and the temperature inside the car rises to 75°C, what is the new volume of the balloon (assuming constant pressure). 15. The scuba diver in the figure blows an air bubble 10 m underwater. As it rises to the surface, the pressure decreases from 2.25 atm to 1.03 atm. What will the volume of air in the bubble at the surface? 16. If 500 g of iron absorbs 22,000 cal of heat, what will be the change in temperature? (specific heat of iron = 0.11 ) 17. A 55.0-g piece of copper wire is heated, and the temperature of the wire changes from 19.0 C to 86.0 C. The amount of heat absorbed is 343 cal. What is the specific heat of copper? 18. A certain substance with a molar mass of 43 g has a heat of fusion of 48 cal/g. How many calories are needed to melt 7.2 kg of the substance? 19. The following length measurements were taken by students using several different measuring devices. Find the average of the measurements. Make sure that your answer has the correct number of significant figures. 10.05 cm, 10.1 cm, 9.741 cm, 10.6 cm, 10.5 cm 20. Round off the measurement 0.003 095 5 m to three significant figures. 21. What is the sum of 2.7 g and 2.47 g expressed in the correct number of significant digits? 22. Perform the following operation: 3.43 cm significant figures. 5.2 cm. Make sure that your answer has the correct number of 23. If a total of 13.5 mol of NaHCO and 4.5 mol of C H O react, how many moles of CO and Na C H O will be produced? 3NaHCO (aq) + C H O (aq) 3CO (g) + 3H O(s) +Na C H O (aq) 24. If 8.6 L of H reacted with 4.3 L of O at STP, what is the volume of the gaseous water collected (assuming that none of it condenses)? 2H (g) + O (g) 2H O(g) 25. The decomposition of potassium chlorate yields oxygen gas. If the yield is 95%, how many grams of KClO are needed to produce 10.0 L of O ? 2KClO (s) 2KCl(s) + 3O (g) 26. How much heat is required to melt 1.6 moles of NaCl ( H = 30.2 kJ/mol) at its melting point? 27. Titanium dioxide (TiO2) is an industrial chemical used as a white pigment in paint. The conversion of volatile TiCl4 to TiO2 occurs according to the reaction, . If 15.70 g of TiCl4 reacts in excess oxygen to form 10.40 g Cl2, what is the percent yield of the reaction? 28. Phosphorus pentachloride is formed when 17.2 g of chlorine gas react with 23.2 g of solid phosphorus (P2). Determine the reactant that is in excess. 29. Calculate the temperature of 2.0 moles of a gas occupying a volume of 5.0 L at 2.46 atm. 30. Calculate the enthalpy change of the reaction CH4 (g) + 2Cl2(g) thermochemical equations: a) C(s) + 2 Cl2 (g) b) CH4 (g) CCl4 (l) C(s) + 2H2 (g) CCl4 (l) + 2 H2 (g), using = –128 kJ = + 75 kJ 31. Use standard enthalpies of formation to calculate the enthalpy change for the following reaction: 4NH3(g) + 7O2(g) 4NO2(g) + 6H2O (l) 32. What is the charge on the cation in CuSO ? 33. Explain the difference between precision and accuracy. Suppose you made three different mass measurements of a sugar sample you knew to have a mass of 1 g. How would you know whether or not the measurements were accurate? How would you know whether or not they were precise? Could the three measurements be precise, but not accurate? Explain. 34. Describe the rules that are used to determine the number of significant figures in the results of addition, subtraction, multiplication, and division. 35. Given the name of a binary molecular compound, describe how to write its formula. Use carbon tetrachloride as an example. 36. Explain how to name binary acids and acids containing a polyatomic ion. Give examples of both. 37. Explain how to write an ionic formula, given an anion and a cation. As an example, use the phosphate anion. Write formulas for the compounds produced by the combinations of these ions. Name the compounds for which you have written formulas. 38. Name the compounds CuBr , SCl , and BaF . Explain the use or omission of the Roman numeral (II) and the prefix di-. 39. What are the 5 main types of chemical reactions? Give an example of each. a. b. c. d. e. 40. What determines whether one metal will replace another metal from a compound in a single-replacement reaction? 41. Predict the precipitate that forms when aqueous solutions of silver nitrate and potassium chloride react to form products in a double-replacement reaction. Include a discussion of how to write the complete chemical equation describing this reaction. 42. The density of acetylene at STP is 1.17 g/L. The empirical formula of acetylene is CH. Describe how you can determine the molar mass of acetylene and its molecular formula if you know only its density at STP and its empirical formula. 43. A reaction is predicted to result in 75.0 grams of product being made, but only 58.3 grams are actually produced. Find the percent yield of this reaction, and explain several reasons why the reaction does not produce as much as predicted. 44. Aluminum metal is burned in oxygen gas. a. Write the balanced equation for this reaction. b. If 5.433 g of aluminum is burned with 8.834 g of oxygen gas, what is the limiting reagent? c. What mass of product can be made