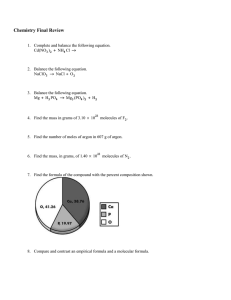
1. 2. 3. 4. 5. (a) Solder is an alloy made of tin and lead that is used in electronic circuits. A certain solder has a melting point of 224 oC. What is its melting point in oF? in K? 435 oF ; 497 K (b) Mercury, the only metal that exists as a liquid at room temperature, melts at – 38.9oC. Convert its melting point to oF -38.02 oF Suppose you are discontented with the existing temperature scale and you decided to formulate a new one in degrees Ang, oA and that on this scale, water boils at 125 oA and freezes at 12.0OA. a) Convert a temperature of 42.0OA to OC. 26.6 O C b) Convert a temperature of 24.0OF to OA. 6.98 O A A piece of silver (Ag) metal weighing 194.3 g is placed in a graduated cylinder containing 242.0 mL of water. The volume of the now reaches 260.5 mL. From these data, calculate the density of silver. 10.50 g/mL The following procedure was used to determine the volume of a flask. The flask was weighed dry and then filled with water. If the masses of the empty flask and filled flask were 56.12 g and 87.39 g, respectively, and the density of water is 0.9976 g/cm3, calculate the volume of the flask in cm3. 31.35 cm3 A cylindrical glass tube 12.7 cm in length is filled with mercury. The mass of mercury needed to fill the tube is 105.5 g. Calculate the inner diameter of the tube in cm. Density of mercury is 13.6 g/mL. 0.882 cm 01. (a) e.g4 Peroxyacylnitratev (PAN) is one of the components of smog. It is a compound of C, H, N and O. Determine its empirical formula from the following percent composition by mass: 19.8 % C, 2.50 % H, 11.6 % N. C2H3NO5 e.g 5 Monosodium glutamate (MSG), a food flavor enhancer, has been blamed for “Chinese Restaurant Syndrome,” the symptoms of which are headaches and chest pains, MSG have the following composition by mass: 35.51 % C, 4.77 % H, 37.85 % O, 8.29 % N and 13.6 % Na. What is the molecular formula if its molar mass is 169 g? NaC5H8NO4 e.g6 When 0.1156 g of a compound, composed of carbon, hydrogen and nitrogen is reacted with oxygen, 0.1638 g CO2 and 0.1676 g H2O are collected. Determine the E.F. of the compound. CH6O e.g7 Terephthalic acid is an important chemical used in the manufacture of polyesters and plasticizers. It contains only C,H and O. Combustion of 19.81 mg of terephthalic acid produced 41.98 mg CO2 and 6.45 mg H2O. The molar mass of Complete the following table: terephthalic acid is 166 g. Calculate the SYMBOL Z A PROTON NEUTRON ELECTRON Sn 50 118 50 68 50 Ag 47 109 47 62 47 Ar 18 40 18 22 18 Cs 55 133 55 78 55 (b) 7.174, what is the natural abundance of the lightest isotope? 79.6 % E.F. and the M.F. of terephthalic acid. EF: C4H3O2 MF : C8H6O4 e.g8 A 0.755 g sample of hydrated copper (II) sulfate (CuSO4 xH2O) was heated carefully until it is changed completely to anhydrous Determine the number of subatomic copper (II) sulfate, CUSO4, with a mass of properties of the element mercury, Hg, 0.438 g. Determine the given the symbol 200 value of x (molecules of water). CuSO4 .6H2O X , 80 e.g9 P = 80 ; E = 80; N = 120 A sample of MgSO4 x H2O weighing 8.129 g is heated until all the water of hydration is 02. The atomic weight of element X is 51.7 u. If element X consists of 2 isotopes that have mass driven off. nos. of 50.0 and 52.0, what is the percent compound, MgSO4, abundance of the lighter isotope? 15.0 % the formula of the hydrate? MgSO4 .7 H2O 03. Naturally occurring element Z, consists of Z-24 (mass is 23.99 u), Z-25 (mass is 24.99 u) and Z-26 (mass is 25.98 u). If the natural abundance of Z25 is 10.0% and the isotope ratio Z-24/Z-26 is 1. The resulting anhydrous weighs 3.967 g. What is A compound of chlorophene (hexachlorophene), used in making germicidal soaps, has the percent composition by mass: 38.37 % C, 1.49 % H, 52,28 % Cl and 7.86 % O. What is the empirical formula 3NaHCO3(aq) + C6H8O7(aq) 3CO2(g) + of this compound? C13H6Cl6O2 2. 3H2O(l) + Na3C6H8O7(aq) A 0.1888 g sample of a hydrocarbon produces 0.6260 g CO2 and 0.1602 g H2O in a combustion analysis. Its molecular mass is found to be 106 g. For this hydrocarbon, determine its: (a) mass percent composition90.0 % C ; 9.50 % (a) What mass of C6H8O7 should be used for every 1.00 x 102 mg of NaHCO3? 79.2 mg (b) What mass of CO2(g) would be produced from such mixture? 78.6 mg e.g How many grams of N2F4 can theoretically be prepared from 4.00 g of NH3 and 14.0 g of F2. H The chemical equation for the reaction is: (b) E.F. C4H5 NH3 + F2 N2F4 + HF 7.66 g (c) M.F. C8H10 3. A certain hydrate is found to have the e.g. H2S + NaOH Na2S + H2O composition 20.3% Cu, 8.95% Si, 36.3% F and 34.5% H2O, by mass. How many grams of Na2S are formed if 2.05 g of What is the empirical H2S is bubbled into a solution containing 1.84 g of formula of the hydrate? CuSiF6 .6H2O 4. NaOH, assuming that the limiting reactant is Determine the empirical formula of benzyprene, a completely consumed? 1.80 g suspected cancer-causing agent found in cigarette smoke and smoke produced in charcoal grilling of meat, which consists of 95.21% C and 4.79% H by e.g by controlled reaction between cyclohexane, Selenium, an element used in the manufacture of C6H12 and O: photoelectric cells and solar energy devices, C6H12 + O2 H2C6H8O4 + H2O forms two oxides. One has 28.8% O by mass and the other 7.8% O. these oxides? Adipic acid, H2C6H8O4, is a raw material used in the production of nylon. It is made commercially mass. C5H3 5. Consider the following reaction: (a) What are the formulas of starting with 25.0 g of cyclohexane and that Propose acceptable names for cyclohexane is the limiting reactant, what is these oxides. SeO2 – selenium dioxide the theoretical yield of adipic acid? 43.5 g SeO3 - selenium trioxide 6. (b) If you obtain 33.5 g of adipic acid from the Para-cresol is used as a disinfectant and in the reaction, what is the percent yield of adipic manufacture of herbicides and artificial food flavors. A 0.4039 g sample of these carbon- hydrogen-oxygen compound yields 1.1518 g CO2 acid? 77.0 % e.g. be the E.F. of para-cresol? C7H8O What is the E.F. of this substance? C8H8O2 or C6H5CO2CH3 e.g The reusable rockets of the U.S. space shuttle use a mixture of aluminum and ammonium per chlorate for fuel. reaction is: AlCl3(s) + 3NO(g) + 6H2O(g) What mass of NH4ClO4 should be used in the fuel mixture for every 1.00 kg of Al? 4.35 kg e.g. Elixirs, such as Alka-Seltzer, use the reaction of sodium bicarbonate with citric acid in aqueous solution to produce a fizz: an important prepared by the reaction between hydroxide: 2C6H5NO2 + 4C6H14O4 C12H10N2 + 4C6H12O4 + 4H2O (a) What is the theoretical yield of azobenzene when 115 g of nitrobenzene and 327 g of triethylenglycol are allowed to react? 85.1 g (b) If the reaction yields 55.0 g of azobenzene, what is the percent yield of azobenzene? 64.6 % A possible equation for the 3Al(s) + 3NH4ClO4(s) Al2O3(s) + is C6H14O4, in the presence of zinc and potassium used in the manufacture of perfumes, is found to 1.252 g oxygen. C12H10N2, nitrobenzene, C6H5NO2 and triethylene glycol, A 5.323 g sample of methyl benzoate, a compound contain 3.758 g carbon, 0.316 g hydrogen and Azobenzene, intermediate in the manufacture of dyes. It can and 0.2694 g H2O in combustion analysis. What is 7. Assuming that you carry out this reaction e.g. The reaction of 13.0 g of C4H9OH, 21.6 g NaBr and 33.8 g of H2SO4 yields 16.8 g of C4H9Br in the reaction: C4H9OH + NaBr + H2SO4 C4H9Br + NaHSO4 + H2O What are the: (a) theoretical yield 24.0 g (b) actual yield 16.8 g (c) per cent yield of the reaction? 70.0 % 2. Many antacids contain aluminum hydroxide, C2H6(g), if all gases are measured at the same Al(OH)3, as their active ingredient. temperature and pressure? 52.5 L (a) Write a balanced equation for the reaction of 2C2H6(g) + 7O2(g) 4CO2(g) + HCl in stomach acid with solid Al(OH)3 to What volume of CO2 is produced? 30.0 L form water and aqueous AlCl3. e.g 14 What volume of phosphine, PH3 (measured at (b) How many grams of HCl reacts with 2.50 g of STP) would be formed by the reaction of 54.6 Al(OH)3? 3.51 g 3. 6H2O(g) g of calcium phosphide with water? 13.0 L Ethylene, C2H4, burns in air: Ca3P2(s) + 6H2O(l) 3Ca(OH)2(s) + 2PH3(g) C2H4 + O2 CO2 + H2O How many grams of CO2 can form when a mixture of 2.93 g of C2H4 and 5.29 g of O2 is ignited, assuming only the reaction above occurs? 4.85 g 4. helium gas has a total pressure of 0.900 atm. amounts of O2 involves the decomposition of What is the partial pressure of oxygen? 0.100 How many grams of O2 can be prepared from 4.50 g of KClO3? 1.76 g atm e.g Consider the reaction: Al + Cl2 AlCl3 volume will this sample occupy dry at STP? 32.9 allowed to react. mL e.g (a) What is the limiting reactant? Al What is the partial pressure of each gas in a mixture which contains 10 mols of He, 2.0 mols (b) How many moles of AlCl 3 are formed? 1.50 of N2 and 0.50 mol of O2 if the total pressure of mol the mixture is 5.0 atm? PHe = 4.0 atm A strip of Zn metal weighing 2.00 grams is placed PN2 = 0.80 atm in an aqueous solution containing 2.50 g of silver PO2 = 0.20 atm nitrate, causing the following reaction to occur: 1. What is the density of fluorine gas at STP? 0.848 2. A gas has a density of 0.992 g/L at 91.0 o and 750 Zn + AgNO3 Ag + Zn(NO3)2 How many grams of Ag will form? 1.58 g 7. 370 ml of oxygen is collected over water at 23.0o and a barometric pressure of 0.992 atm. What A mixture of 1.50 mol of Al and 3.00 mol of Cl 2 is 6. A mixture of 40.0 g of oxygen and 40.0 g of A common laboratory method for preparing small KClO3. 5. e.g g/L Automotive airbags inflate when sodium azide, torr. What is the molecular weight of the gas? NaN3, rapidly decomposes to the elements. 30.02 g/mol (a) Write a balanced equation for this reaction? 3. (b) How many grams of NaN3 are required to form A certain gas occupies a volume of 100 ml at a temperature of 29.0oC. What will be its volume at 1.00 g N2? 1.62 g 10.0oC if the pressure remains constant? 93.7 mL (c) How many grams of NaN3 are required to 4. 750 ml of a gas at 300 torr pressure and 50.0 oC is produce 12.0 ft3 of N2 if the gas has a density heated until the volume of the gas is 2000 ml at a of 1.25 g/L? 1.32 x 103 g pressure of 700 torr. What is the final temperature of the gas? 1737 oC e.g The ratio of the rate of effusion of gas X to 5. the rate of effusion of N2(g) is rx L steel cylinder if the pressure is 10.0 atm and rN2 What is the molecular weight of gas X if it e.g the temperature is 27.0oC? 20.3 mols 6. 66.0 g of He gas are pumped into a 0.500 L effuses 0.876 times as rapidly as N2? 36.4 cylinder at 60.0oC. What is the pressure of the g/mol gas in the cylinder? The diffusion rate of an unknown gas is 7. Suppose 100 ml of oxygen is collected over water measured and found to be 31.50 ml/min. in the laboratory at a pressure if 700 torr and a Under identical experimental conditions the temperature of 20.0oC, what would the volume of diffusion rate of O2 is found to be 30.50 dry oxygen be at STP? 83.66 mL ml/min. which of the following is the unknown 8. gas? CH4, CO, NO, CO2 or NO2? MW = 30.0 What volume of oxygen is required for the complete combustion of 15.0 L of ethane, Calculate the volume of oxygen necessary to burn 50.0 L of CO. 25.0 L g/mol, gas is NO e.g How many mols of hydrogen are present in a 50.0 2CO + O2 2CO2 9. A container is filled with gas to a pressure of 5.00 atm at 30.0oC. a) What pressure will develop inside the sealed container when it is warmed to 100oC? 08. A commercial bleaching solution contains 3.62 6.16 mass percent sodium hypochlorite, NaOCl. What atm is the mass of NaOCl in a bottle containing 2500. b) At what temperature would the pressure be 100 atm? 5.79 x 10 3 o g of bleaching solution? What C be its concentration expressed as mole fraction NaOCl? 10. What volume will 10.0 g of CO occupy at STP? Assume CO us an ideal gas. 8.00 L 11. will XNaOCl = 0.00903 09. What is the mole fraction of naphthalene, C10H8 in o A 500 ml sample of gas weighs 0.326 g at 100 C a solution made by dissolving 36.5 g naphthalene in and 380 torr. 420 g toluene, C7H8? 0.0588 What is the molecular weight of the gas? 39.9 g/mol 10. A solution containing 46.0 g acetone, C3H6O, and 12. Find the density of ammonia, NH3, at 100oC when 66.0 g of H2O, has a density of 0.926 g/ml. confined by a pressure of 1600 mm Hg. 1.17 g/L Calculate: (a) the mass percentage 69.7 % 13. The time required for a given volume of N2 to (b) the mole fraction Xacetone = 0.178 effuse through an orifice is 35.0 sec. Calculate Xwater = 0.822 the MW of the gas which requires 50.0 sec to (c) the molality 12.0 m effuse through the same orifice under the same (d) the molarity of the solution 6.55 M conditions. 57.1 g/mol 14. A cylinder contains 36.0 g He gas, 140.0 g of N2 gas and 264.0 g of CO2. e.g.1 A lamp draws a current of 3.00 amperes. Find the charge in coulombs used by the lamp in 60.0 a) What is the mole fraction of He? 0.450 secs. 180 coulombs b) The total pressure of the cylinder is given at 15.0 atm. Compute for the partial pressure if e.g.2 the He gas in the atmosphere. 6.75 atm A dynamo delivers 25.0 amperes at 220 volts. (a) Determine the power in kilowatts supplied by 01. A solution is prepared by dissolving 25.0 ml the dynamo. (b) How much electrical energy in kilowatt- ethanol, C2H5OH (d = 0.789 g/ml), in enough water hour, to produce 250.0 ml solution. hours? What is the molarity of ethanol in the solution? 1.71 M 5.50 kW is supplied by the dynamo in 3.50 19.3 kW-hr (c) What is the cost of energy at P 7.9207 02. A 22.3 g sample of acetone, (CH3)2CO, is dissolved per kilowatt-hour? Php 152.47 in enough water to produce 125 ml solution. What is the molarity of acetone in this solution? 3.08 m e.g1 What weights of gold and chlorine will be 03. How would you prepare 425 g of an aqueous formed when 10,000 coulombs of electricity is solution containing 2.40 % by mass of sodium passed through a water solution of gold (III) acetate, NaC2H3O2? Dissolve 10.2 g NaC2H3O2 in chloride? The electrode reactions are: 414 g H2O 04. A particular analytical chemistry Au3+ + 3e- Au 6.80 g procedure requires 0.0500 M K2CrO4. What volume of 0.250 M K2CrO4 must we dilute with water to prepare 0.100 L of 0.0500 M K2CrO4? 0.0200 L 05. Lauryl alcohol, C12H25OH, is prepared 2Cl- Cl2 + 2ee.g2 2.50 amp. How many grams of Cu plate out in 30.0 min? 1.48 g from coconut oil; it is used to make sodium lauryl sulfate, a synthetic detergent. What is the molality of lauryl alcohol in a solution containing e.g3 06. What mass of iodine, I2, in grams, must be dissolved in 315.0 ml of carbon disulfide, CS 2 (d = 1.261 g/ml), to produce a 0.182 m solution? 18.3 g 07. An aqueous solution is 36.0 % HNO3 by mass and has a density of 1.2205 g/ml. What is the molarity and molality of this solution? 6.97 M ; 8.93 m How many minutes will it take to plate out 58.7 g of Ni from a solution of NiSO4, using a current of 0.900 amp? 3.57 x 104 min 15.6 g of lauryl alcohol dissolved in 125 g of ethyl alcohol, C2H5OH? 0.671 mol/kg A Cu2+ solution is electrolyzed using a current of e.g4 If 0.690 g of Ag is deposited on the cathode of a silver coulometer (a) the How many coulombs have passed through circuit? 617 coulombs (b) If the process takes 20 minutes, what was the rate of the current? 0.514 A e.g5 e.g6 How many grams of Ca metal could be produced (a) the expression for the approximate amount by the electrolysis of molten CaBr2 using a of the radioactive material present at any current of 30.0 amp for 10.0 hours? 224 g time t. N = No e-0.0527t/yr (b) the mass of the material after 5 years. 38.4 How many seconds will be required to produce mg 1.0 g silver metal by the electrolysis of a AgNO 3 (c) the time at which the material decayed to solution using a current of 30 amperes? 29.8 sec e.g 7 one half of its initial mass 13.2 years 3. Carbon-14 is one of the isotopes of carbon, with a half-life of 5,730 years. Find the decay constant for this element. 1.209 x 10-4 / year What is the equivalent weight of the metal if the current of 0.300 amp will cause 0.129 g of the metal to plate out of a solution undergoing electrolysis in 40.0 minutes? 17.3 g 4. The half-life of hafnium-156 is 0.0250 s. How long will it take a 560. g sample to decay to one-fourth its original mass? 0.0500 s 1. A certain radioactive material weighing 500. g is found to be 420. g after 30.0 days. How long would it take the substance to be 100. g? 277 days 2. Radium decomposes at a rate proportional to the quantity of radium present. Suppose that it is found that in 25.0 years approximately 1.10 % of a certain quantity of radium has decomposed. Determine approximately how long will it take for ½ the original amount of radium to decompose. 1.57 x 10 3 years 3. A certain radioactive substance has a half-life of 38.0 hours. Find how long it takes for 90.0 % of the radioactivity to be dissipated. 127 hours 4. The half-life of a material is 7.50 years. How much of a 3.00 mg sample will be left after a 20.0year period? 0.473 mg 5. Carbon-11, used in medical imaging has a half-life of 20.4 minutes. formed and then The carbon-11 nuclides are incorporated into desired compounds. The resulting sample is injected into a patient and the medical image is obtained. The entire process takes five half- lives. What percent of the original carbon-11 remains at this time? 3.12 % 1. Starting with 50 grams of strontium-90, 40.45 grams remain after a five-year period. (a) What is the half-life of strontium? 16.3 years (b) How much strontium-90 will remain after 12 years? 30 g 2. A certain radioactive material is known to decay at a rate proportional to the amount present. If initially there are 50 mg of the material and after 2 hours it is observed that the material has lost 10 % of its original mass, find, 5. Gold-198 has a half-life of 2.70 days. How much of a 96.0 g sample of gold-198 will be left after 8.10 days? 12.0 g