Synthesis of Salicylic Acid
advertisement

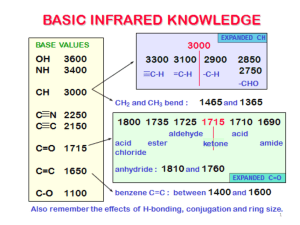
Dirk Emde Mark Rosen November 12, 2010 CHM 2210 Molecular Weight: 138.12 g/mol Chemical Formula: (HO)C6H4(COOH) Appearance: Fine, white crystals. Odor: Odorless or slight phenolic odor. Density: 1.44 g/mL @ 20°C (68°F) pH: 2.4 Boiling Point: 211°C (412°F) Melting Point: 157 - 159°C (315 - 318°F) Sublimes @ 76°C (169°F) Warning! Harmful if swallowed, inhaled or absorbed through skin. Affects central nervous system, kidneys, and pancreas. Causes irritation to skin, eyes and respiratory tract. Oral rat LD50: 891 mg/kg IUPAC name: 2hydroxybenzoic acid Arene Ring Carboxylic Acid group off carbon 1 Alcohol group off carbon 2 0 stereocenters Prochiral 3500–3200 O–H stretch 3300–2500 O–H stretch carboxylic acids 3100–3000 C–H stretch aromatics 1760–1690 C=O stretch carboxylic acids 1600–1400 C–C stretch (in–ring) aromatics Acetylsalicylic acid is marketed as aspirin. Best known for its use in antiacne treatments. Written about by Hippocrates in the 5th century BC. First isolated and named by the German chemist Johann Andreas Buchner in 1826. 1. 2. 3. Nucleophilic addition of OH- to ester carbonyl group forms alkoxide intermediate. Water receives H from OH and forms hydronium Elimination of alkoxide ion forms carboxylic acid 4. Alkoxide ion is protonated via hydrogen from carboxylic acid and forms carboxylate ion. 5. Electronegative oxygen protonated via Description of isMech prior created hydronium, forming carboxylic acid and reforming alcohol. We’d like to give special thanks to Professor Becker for assistance with mechanism. Thanks to Simon Tang from the tutoring center for help with layout of presentation Dirk Emde Mark Rosen 1. 2. 3. 4. Baker, J.T., Material Safety Data Sheets. www.jtbaker.com/europe/msds/ 1997-2000 Mallonckrodt Baker inc. Diarmuid, Jeffreys. (2005) Aspirin. The remarkable story ofa wonder drug. New York, Ny: Bloomsbury pp. 38-40 McMurry, John Organic Chemistry 7th Edition Cengage Learning Inc. Belmont, Ca. 2008 Spectral Database for Organic Compounds. http://riod01.ibase.aist.gojp/sdbs/cgibin/cre_index.c gi?lan g=eng. Last updated March 2010 AIST. Japan