Reading the Periodic Table
advertisement

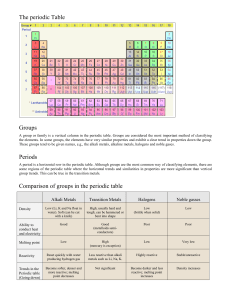
Science Starter: •Get notes from front.Periodic Table on RIGHT side. Notes on LEFT side. Update table of contents: 14 Periodic Table 1.2 15 Periodic Table Families 1.3 •Send someone from group up to get ALIEN Periodic Table. Science Starter: Go to my website and click on science starter article for TUESDAY! SAVE to desktop-if no laptop see me for paper copy If absent yesterday see me! Update table of contents: 14 Periodic Table 1.2 15 Periodic Table Families 1.3 Today’s Objectives: Read closely to learn structure of Periodic Table and begin Color coding PT Science Starter: Elements in a group have… a. A wide range of chemical properties b. The same atomic radius c. Similar chemical properties d. The same number of protons •Go to WED on my WEBSITE if you want to follow with PowerPoint OPEN ISN to 14 PERIODIC TABLE Today’s learning objective•Understand the organization of PT and explain patterns of periods & groups Reading the Periodic Table Lesson 1.2 A way of organizing & classifying elements • Dmitri Mendeleev-Russian chemist • Arranged in rows and columns based on repeating patterns of properties & atomic number • Color of chemical symbol indicates state of element at room temperature-white=gas, blue=liquid, black=solid • Background color=whether element is metal, nonmetal, metalloid Columns • The vertical (up and down) columns of the periodic table (there are 18) are called groups or families. • Elements in the same group or family have similar characteristics or properties. Rows • The horizontal rows of the periodic table are called periods. • Elements in a period are not alike in properties. • The first element in a period is usually an active solid, and the last element in a period is always an inactive gas. Rows • Atomic size (number of protons) decreases from left to right across a period. • Atomic mass (number of protons) increases from left to right across a period. Rows • Metals are on the left • Non-metals are on the right Elements that have not yet been named have temporary 3 letter symbols Color the square for Hydrogen pink. Lightly color all metals yellow. Place black dots in the squares of all alkali metals. 1 ALKALI METALS •very reactive metals that do not occur freely in nature •malleable, ductile, good conductors of heat and electricity. •Low density; softer than most other metals (can be cut w/ a knife) •can explode if they are exposed to water •1 outer electron •Used in soap, fertilizers, and medicine Draw a black triangle in each box in the group of alkaline earth metals. ALKLINE EARTH METALS •not found free in nature •Metals; Silver colored •very reactive (but less reactive than alkali metals) •2 outer electrons •Used in electronics, antacids, fireworks, x-rays and metal alloys Draw black diagonal line across element box of transition metals! TRANSITION METALS Groups 3-12 •ductile and malleable, shiny, and conduct electricity and heat •iron, cobalt, and nickel, are the only elements known to produce a magnetic field. •1 or 2 outer electrons •Used in jewelry, wires, coins, metal alloys RARE EARTH ELEMENTS: Lanthanides and Actinides Shade these in RED. •many are man-made •Transitions metals-placed at bottom so not too wide •Lanthanides: shiny & reactive •Actinides: ALL RADIOACTIVE & unstable •Elements 95-103 are manufactured in a lab OTHER METALS •are ductile and malleable •are solid, have a relatively high density, and are opaque •They are part of groups 13-16 METALLOIDS: Trace zigzag line in black than color purple B. Si, Ge, As, Sb, Te, Po, At •have properties of both metals and nonmetals •some of the metalloids, such as silicon and germanium, are semi-conductors. This means that they can carry an electrical charge under special conditions. This property makes metalloids useful in computers and calculators NON-METALS color these elements green •not able to conduct electricity or heat very well •very brittle, and cannot be rolled into wires or pounded into sheets •exist in two of the three states of matter at room temperature: gases (such as oxygen) and solids (such as carbon). •have no metallic luster, and do not reflect light. Group 13: BORON FAMILY uses: cooking, LCD displays, poisons Group 14: CARBON FAMILY uses: graphite, diamonds, plumbing Group 15: NITROGEN FAMILY uses: refrigerant, make-up, medicines Group 16: OXYGEN FAMILY uses: medicines, water treatment, nukes, fungicide, printers, solar panels HALOGENS: Color these ORANGE •"halogen" means "salt-former" and compounds containing halogens are called "salts" •7 outer level electrons •Nonmetals, very reactive •exist in all three states of matter: •Solid- Iodine, Astatine •Liquid- Bromine •Gas- Fluorine, Chlorine USED in toothpaste, water treatment, thyroid medicines NOBLE GASES: Color these in BLUE •Inert gases; unreactive •Nonmetals •Full valence electrons so do NOT bond with others •Found in lights, balloons, electronics