Concept of Heat
advertisement

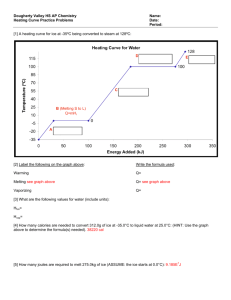
INTRODUCTION When you heat a substance, you are transferring energy into it by placing it in contact with surroundings that have a higher temperature P-26 SC TEAM OBJECTIVES define and distinguish between various units of heat define the mechanical equivalent of heat discuss everyday examples to illustrate these concepts WHAT IS HEAT? Heat is energy that flows from a higher temperature object to a lower one because of difference in temperature. Heat can change the state of substances. When heated UNIT OF HEAT Heat is measured in unit of calorie. One calorie is defined as the amount of the heat energy required to raise the temperature of 1ºC of 1 gram of water. It will take 500 calories to heat 500 grams of water from 20ºC to 21ºC. In SI, heat is measured in unit of joule. One calorie is equal to 4,184 joule, and it is frequently rounded up to 4,2 joule. Unit of HEAT Heat is measured in Joule (J) or calorie (cal). 1 Joule = 0,24 calorie 1 calorie = 4,186 Joule. 1 kilo calorie = 1 kcal = 4.186 Joule. If an object has become hotter, it means that it has gained heat energy. If an object cools down, it means it has lost energy MECHANICAL EQUIVALENT OF HEAT Joule demonstrated that water can be heated by doing (mechanical) work, and showed that for every 4186 J of work done, the temperature of water raise by 1⁰C per kg. THE THERMAL ENERGY (Q) Specific heat capacity (J / kg C) = Q m c T Mass of object (kg) Thermal energy (J) The temperature Change (C) ΔT = T final - Tinitial THE AMOUNT OF THERMAL ENERGY (Q) Greater amount of heat is required to raise the temperature of greater mass of the material. The amount of thermal energy is influenced by: Mass Type of object Temperature change The bigger mass the bigger thermal energy. The difference object has difference thermal energy. The higher temperature change, the higher thermal energy. If ΔT is positive, Q is also positive. It means that a substance has an increase in temperature and gets the heat energy (receives the heat). If ΔT is negative, Q is also negative; it means that the substance has a decrease in temperature and will lose the heat energy (releases the heat). EXAMPLE What is the amount of heat required to raise the temperature of 2 kg of copper from 30 C to 80 C, given the specific heat capacity of cooper is 400 J / kg C ? Solution: m = 2 kg T = 80 C – 30 C = 50 C c = 400 J / kg C Q = m c T = 2 kg x 400 J / kg C x 50 C = 40 000 J So, the amount of heat is 40 000 J A silver spoon has a mass of 32 g and then it is cooled from 60ºC down to 20ºC. How much heat is released by the spoon? given the specific heat capacity of silver is 235 J/(kgºC) EXAMPLE A silver spoon has a mass of 32 g and then it is cooled from 60ºC down to 20ºC. How much heat is released by the spoon? Known: m = 32 g = 0.032 kg c = 235 J/(kgºC) TInitial = 60ºC Tfinal = 20ºC ΔT = Tf – Ti = -40ºC Question Q = ..... ? Solution: Q = m . c . Δ T = 0.032 kg. 235 J/(kgºC). -40ºC = - 301 J spoon releases the heat of 301 J. So that the spoon becomes cooler. Heat Capacity (C) Temperature change by T C Heat capacity (C) is defined as the amount of thermal energy required to raise the temperature of body by 1 K or 1 C. C= Q T Specific Heat Capacity (c) The amount of thermal energy needed by 1 kilogram of substance to increase its temperature up to 1 Kelvin is called specific heat capacity m kg Temperature changes byT C c= Q m T SPECIFIC HEAT CAPACITY OF SOME MATERIALS Material Aluminum Brass Cooper Ice Iron Lead Specific Heat capacity (J / kg C ) 920 380 400 2100 460 130 Material Steam Mercury Methylated spirit Sea water Water Zinc Specific Heat capacity (J / kg C ) 2010 140 2400 3900 4200 390 Water and other substances that have high specific heat capacity can absorb more heat energy with less change of temperature. LATENT HEAT (L) AND SPECIFIC LATENT HEAT Latent heat of fusion, (Lf) is heat per unit mass needed to change a substance from solid to liquid without change in temperature. Lf = Q m Q = m Lf Latent heat of vaporization, (LV) is heat per unit mass needed to change a substance from liquid to solid without change in temperature. LV = Q m Q = m LV PHASES OF MATTER Heat required for phase changes: Vaporization: liquid vapour Melting: solid liquid Sublimation: solid vapour Heat released by phase changes: Condensation: vapour liquid Fusion: liquid solid Deposition: vapour solid PHASES OF MATTER GRAPH OF PHASE CHANGES Useful data for thermal energy calculation Specific latent heat of water = 336 000 J/kg Specific latent heat of steam = 2 260 000 J/kg Specific heat capacity of water = 4200 J/kg C Specific heat capacity of ice = 2100 J/kg C Specific heat capacity of water = 1 cal/gram C Specific heat capacity of ice = 0,5 cal/gram C Example: What is the amount of thermal energy required to change the state of 1 gram of ice at – 30 C to steam at 120 C? Solution: The figure below indicates the experimental results obtained when energy is gradually added to the ice. Let us examine each portion of the red curve. T (C) 120 D 100 C Steam Water + steam B 0 A Ice + Water Water - 30 Ice E Energy added (J) Part A: On this portion of the curve, the temperature of the ice changes from - 30. °C to 0°C. The specific heat of ice is 2100 J / kg C. Q = m ice c ice T = (1 x 10 -3 kg) (2100 J/kg C) (0 C – (-30 C)) = 63 J Part B: When the temperature of the ice reaches 0.0°C, the ice–water mixture remains at this temperature - even though energy is being added - until all the ice melts. Q = m ice Lf = (1 x 10-3 kg) (336 000 J/kg) = 336 J Part C: Between 0.0°C and 100.0°C, nothing surprising happens. No phase change occurs, and so all energy added to the water is used to increase its temperature. The amount of energy necessary to increase the temperature from 0.0°C to 100.0°C is: Q = m water c water T = (1 x 10-3 kg) ( 4 200 J/kg °C) (100 °C) = 420 J Part D: At 100.0°C, another phase change occurs as the water changes from water at 100.0°C to steam at 100.0°C. Q = m water LV = (1 x 10-3 kg) (2 260 000 J/kg) = 2 260 J Part E: On this portion of the curve, as in parts A and C, no phase change occurs; thus, all energy added is used to increase the temperature of the steam. The energy that must be added to raise the temperature of the steam from 100.0°C to 120.0°C is Q = m steam c steam T = (1 x 10-3 kg) (2 010 J/kg °C) (120 – 100 °C) = (1 x 10-3 kg) (2 010 J/kg °C) (20 °C) = 40.2 J The total amount of energy that must be added to change 1 gram of ice at - 30.0°C to steam at 120 0°C is the sum of the results from all five parts of the curve. Q = QA + QB + QC + QD + QE = 63 J + 336 J + 420 J + 2 260 J + 40.2 J = 3119.2 J CALORIMETERS BLACK’S PRINCIPLE BLACK’S PRINCIPLE