Document
advertisement
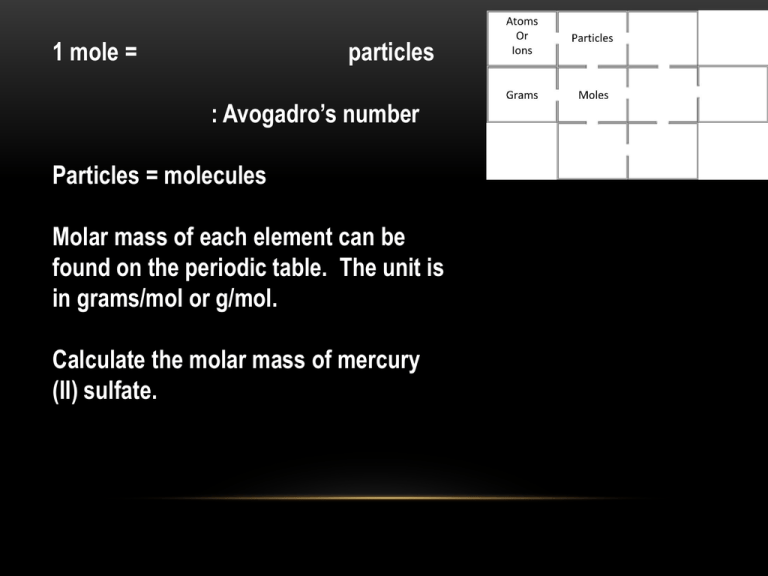
1 mole = particles : Avogadro’s number Particles = molecules Molar mass of each element can be found on the periodic table. The unit is in grams/mol or g/mol. Calculate the molar mass of mercury (II) sulfate. p.326. #31, A sample of sodium sulfite (Na2SO3) has a mass of 2.25 g. (a) How many Na+ ions are present? (b) How many SO3-2 ions are present? (c ) What is the mass in grams of one formula unit of Na2SO3? • #33, A sample of ethanol (C 2H5OH) has a mass of 45.6 g. (a) How many hydrogen atoms does the sample contain? • Which has the larger percent by mass of sulfur, H 2SO3 or H2S2O8? • Percent formula = • Empirical formula is the __________ ratio of elements in a compound. • Ionic compound: Is the empirical formula the correct formula? (please do not answer and let the rest of the class some time to think) • Molecular compound: is the empirical compound the correct formula? • The relationship of molecular formula and empirical formula • Determine the molecular formula for ibuprofen. Analysis of ibuprofen yields a molar mass of 206 g/mol and a percent composition of 75.7% C, 8.80% H and 15.5% O. • Combustion reaction: combustion, burns • You need hydrocarbon O 2 • The process produces CO 2 and H2O, both in __________ state • Write the balanced equation of the combustion of liquid C 6H13OH • Synthesis: synthesis, combination, composition • Simple → complex or elements → compound • Write the balanced synthesis equation of magnesium bromide • Decomposition: decomposition • Complex → simple or compound → elements • Write the balanced decomposition equation of magnesium bromide. • Single Replacement Reaction • Metal(A) + Ionic solution → Ionic solution + Metal(B) • Write the balanced equation of nickel added to the solution of magnesium chloride. • Double Replacement • Solution + solution → cations change partners (thoughts: can a cation change partner with an anion?) • Write the balanced equation of adding the solution of sodium phosphate into the solution of manganese (II) chloride. • Write complete ionic equation. • Write net ionic equation. • Dissociation equation: • Solid ionic compound is put into water. Ionic compound will dissociate into cations and anions. • Write the balanced equation of when calcium iodide is put into water.





