+ O 2(g) → CO 2(g)
advertisement
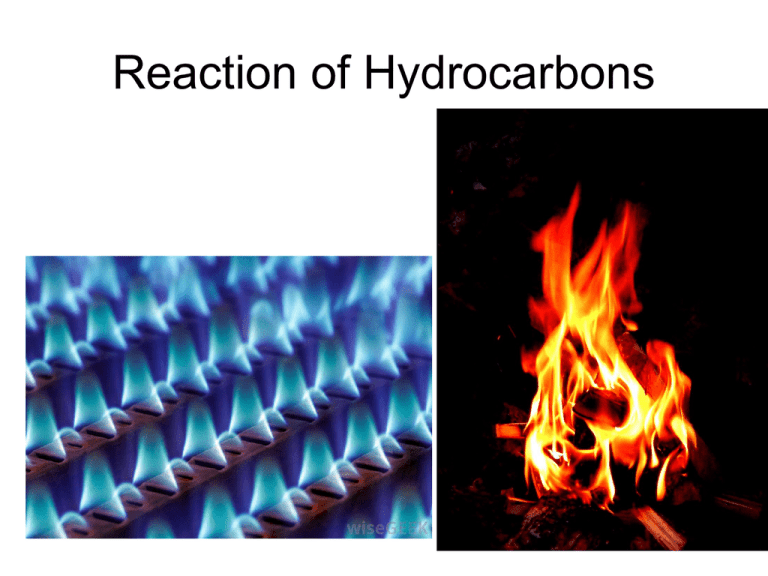
Reaction of Hydrocarbons Addition Reactions A common type of reaction is an _______________ addition reaction of alkenes and alkynes Not alkanes double and ______ triple Where there are _______ bonds, the molecule does not contain the hydrogen maximum number of __________ and is ____________ unsaturated Addition Reactions double Molecules are added across the ________ triple bond or ______ Addition Reactions Addition Reactions Addition Reactions Minor Major Which product do we get? Markovnikov’s Rule When adding a binary acid (like HCl) to an asymmetric alkene, the hydrogen goes to the carbon with more __________ hydrogens The rich get richer ___________________ Combustion Reactions Another common reaction is combustion If there is sufficient oxygen, then we have _________ complete combustion 1 C3H8(g) + 5 O2(g) → 3 CO2(g) + 4 H2O(g) Balancing Complete Combustion Example Write and balance the equation for the complete combustion of acetylene (ethyne). Formula for ethyne is:C2H2 Reacts with oxygen gas: O2 Products are carbon dioxide and water: CO2 H2O Balance! 2 C2H2(g) + 5 O2 (g) 4 CO2 (g) + 2 H2O (g) Combustion Reactions If there is insufficient oxygen, then we incomplete combustion have ___________ Instead of only carbon dioxide and water as products, we also form carbon _______________ monoxide Carbon (soot) and _______________ Hydrocarbon(g) + O2(g) → CO2(g) + H2O(g) + CO(g) + C(s) Incomplete Combustion Reactions Incomplete combustion occurs when there is not enough oxygen present to fully burn up the hydrocarbon. The yellow flame of a candle indicates incomplete combustion because it is releasing carbon, which emits light energy in the yellow range of the visible spectrum. An incomplete combustion reaction can produce other compounds, often toxic. 2 C3H8 (g) + 7 O2 (g) 2 C(s) + 2 CO(g) + 2 CO2 (g) + 8 H2O (g) Balancing Incomplete Reactions Example 2,2,4-trimethylpentane is a major component of gasoline. Write a possible equation for the incomplete combustion reaction. Formula for 2,2,4-tmp is: C8H18 Mixes with oxygen gas: O2 Products are carbon, carbon monoxide, carbon dioxide, and water: C, CO, CO2, H2O Balance! 2 C8H18 (g) + 15 O2 (g) 8 C(s) + 4 CO(g) + 4 CO2(g) + 18 H2O(l)







